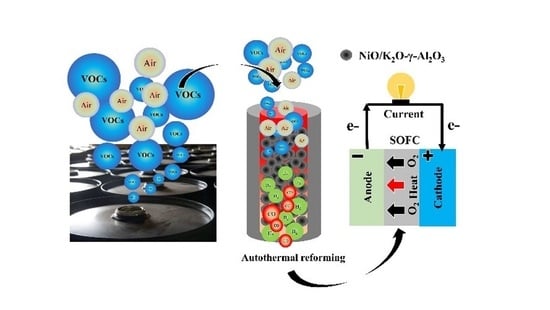
Autothermal Reforming of Volatile Organic Compounds to Hydrogen-Rich Gas
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermodynamic Analysis
2.2. Performance of ATR over Ni-Based Catalyst
2.3. Effect of Operating Conditions on ATR Performance
2.3.1. Effect of Temperature
2.3.2. Effect of O2/C
2.3.3. Effect of S/C
2.3.4. ATR of Real VOCs
2.4. The Efficiency of ATR
3. Experiment and Analysis
3.1. Experiment Setup and Analysis
3.2. Thermal Efficiency of Autothermal Reforming Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, B.; Ji, J.; Zhang, B.; Huang, W.; Gan, Y.; Leung, D.Y.; Huang, H. Catalytic ozonation of VOCs at low temperature: A comprehensive review. J. Hazard. Mater. 2022, 422, 126847. [Google Scholar] [CrossRef] [PubMed]
- Simayi, M.; Hao, Y.; Li, J.; Shi, Y.; Ren, J.; Xi, Z.; Xie, S. Historical volatile organic compounds emission performance and reduc tion potentials in China’s petroleum refining industry. J. Clean. Prod. 2021, 292, 125810. [Google Scholar] [CrossRef]
- Song, S.; Zhang, S.; Zhang, X.; Verma, P.; Wen, M. Advances in Catalytic Oxidation of Volatile Organic Compounds over Pd-Supported Catalysts: Recent Trends and Challenges. Front. Mater. 2020, 7, 595667. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Hsu, S.-K.; Chou, M.-S. Volatile organic compound emissions from wastewater treatment plants in Taiwan: Legal regulations and costs of control. J. Environ. Manag. 2008, 88, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Simayi, M.; Deng, Y.; Xie, S. Spatial-temporal variations and reduction potentials of volatile organic com pound emissions from the coking industry in China. J. Clean. Prod. 2019, 214, 224–235. [Google Scholar] [CrossRef]
- Li, Q.; Su, G.; Li, C.; Wang, M.; Tan, L.; Gao, L.; Mingge, W.; Wang, Q. Emission profiles, ozone formation potential and health-risk assessment of volatile organic compounds in rubber footwear industries in China. J. Hazard. Mater. 2019, 375, 52–60. [Google Scholar] [CrossRef]
- Zheng, C.; Shen, J.; Zhang, Y.; Huang, W.; Zhu, X.; Wu, X.; Chen, L.; Gao, X.; Cen, K. Quantitative assessment of industrial VOC emissions in China: Historical trend, spatial distribution, uncertainties, and projection. Atmos. Environ. 2017, 150, 116–125. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Or ganic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Song, M.; Liu, X.; Zhang, Y.; Shao, M.; Lu, K.; Tan, Q.; Feng, M.; Qu, Y. Sources and abatement mechanisms of VOCs in south ern China. Atmos. Environ. 2019, 201, 28–40. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, Y.; Li, X.; Zhuang, G.; Wang, K.; Zheng, Y.; Sun, D.; Huang, J.; Li, Q. Catalytic benzene oxidation by biogenic Pd nanoparticles over 3D-ordered mesoporous CeO2. Chem. Eng. J. 2019, 362, 41–52. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Zeng, S.; Chen, J. Forecasting the Allocation Ratio of Carbon Emission Allowance Currency for 2020 and 2030 in China. Sustainability 2016, 8, 650. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Ghoshal, A.K. Removal of Volatile Organic Compounds from polluted air. J. Loss Prev. Process Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Ghorbani, B.; Abedi, H. Biodiesel production integrated with glycerol steam reforming process, solid oxide fuel cell (SOFC) power plant. Energy Convers. Manag. 2020, 206, 112467. [Google Scholar] [CrossRef]
- Cui, X. Thermodynamic analysis of steam reforming and oxidative steam reforming of propane and butane for hydrogen production. Int. J. Hydrog. Energy 2018, 43, 13009–13021. [Google Scholar] [CrossRef]
- Qi, A.; Thurgood, C.; Peppley, B. Hydrogen Production for SOFCs Application via Autothermal Reforming of Volatile Or ganic Compounds on Ru-pyrochlore Catalysts. Energy Procedia 2012, 29, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Jirátová, K.; Kovanda, F.; Ludvíková, J.; Balabánová, J.; Klempa, J. Total oxidation of ethanol over layered double hydroxide-related mixed oxide catalysts: Effect of cation composition. Catal. Today 2016, 277, 61–67. [Google Scholar] [CrossRef]
- Puértolas, B.; Smith, A.; Vázquez, I.; Dejoz, A.; Moragues, A.; Garcia, T.; Solsona, B. The different catalytic behaviour in the propane total oxidation of cobalt and manganese oxides prepared by a wet combustion procedure. Chem. Eng. J. 2013, 229, 547–558. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S.; Haryanto, A. Hydrogen production from glycerin by steam reforming over nickel catalysts. Renew. Energy 2008, 33, 1097–1100. [Google Scholar] [CrossRef]
- Czernik, S.; French, R.; Feik, C.; Chornet, E. Hydrogen by Catalytic Steam Reforming of Liquid Byproducts from Biomass Thermoconversion Processes. Ind. Eng. Chem. Res. 2002, 41, 4209–4215. [Google Scholar] [CrossRef]
- Amphlett, J.; Evans, M.; Jones, R.; Mann, R.; Weir, R.D. Hydrogen production by the catalytic steam reforming of methanol part 1: The thermodynamics. Can. J. Chem. Eng. 1981, 59, 720–727. [Google Scholar] [CrossRef]
- Chen, H.; Yu, H.; Peng, F.; Yang, G.; Wang, H.; Yang, J.; Tang, Y. Autothermal reforming of ethanol for hydrogen production over perovskite LaNiO3. Chem. Eng. J. 2010, 160, 333–339. [Google Scholar] [CrossRef]
- Cao, C. A comparative study of Rh and Ni coated microchannel reactor for steam methane reforming using CFD with de tailed chemistry. Chem. Eng. Sci. 2015, 11, 276–286. [Google Scholar] [CrossRef]
- Burns, D.; Piccardi, G.; Sabbatini, L. Some people and places important in the history of analytical chemistry in Italy. Mi Crochim Acta 2008, 160, 57–87. [Google Scholar] [CrossRef]
- Araki, S.; Hino, N.; Mori, T.; Hikazudani, S. Durability of a Ni based monolithic catalyst in the autothermal reforming of bio gas. Int. J. Hydrog. Energy 2009, 34, 4727–4734. [Google Scholar] [CrossRef]
- Balakotaiah, V.; Sun, Z.; West, D. Autothermal reactor design for catalytic partial oxidations. Chem. Eng. J. 2019, 374, 1403–1419. [Google Scholar] [CrossRef]
- Shi, L.; Bayless, D.; Prudich, M. A CFD model of autothermal reforming. Int. J. Hydrog. Energy 2009, 34, 7666–7675. [Google Scholar] [CrossRef]
- Jimmy, U.; Mohamedali, M.; Ibrahim, H. Thermodynamic Analysis of Autothermal Reforming of Synthetic Crude Glycerol (SCG) for Hydrogen Production. ChemEngineering 2017, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, X.; Li, M.; Li, S.; Wang, S.; Ma, X. Thermodynamic analysis of hydrogen production from glycerol autother mal reforming. Int. J. Hydrog. Energy 2009, 34, 5683–5690. [Google Scholar] [CrossRef]
- Sahraei, O.A.; Desgagnés, A.; Larachi, F.; Iliuta, M. Ni-Fe catalyst derived from mixed oxides Fe/Mg-bearing metallurgical waste for hydrogen production by steam reforming of biodiesel by-product: Investigation of catalyst synthesis parameters and temperature dependency of the reaction network. Appl. Catal. B Environ. 2020, 279, 119330. [Google Scholar] [CrossRef]
- Aguilera, D.; Perez, A.; Molina, R.; Moreno, S. Cu–Mn and Co–Mn catalysts synthesized from hydrotalcites and their use in the oxidation of VOCs. Appl. Catal. B Environ. 2011, 104, 144–150. [Google Scholar] [CrossRef]
- Palma, C.F. Modelling of tar formation and evolution for biomass gasification: A review. Appl. Energy 2013, 111, 129–141. [Google Scholar] [CrossRef]
- Ruhswurmova, N.; Kim, S.; Yoo, J.; Chun, D.; Rhim, Y.; Lim, J.; Kim, S.; Choi, H.; Lee, S. Nickel supported on low-rank coal for steam reforming of ethyl acetate. Int. J. Hydrog. Energy 2018, 43, 15880–15890. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, J.; Gao, A.; Zhouyu, M.; Liu, B.; Gao, M.; Zhang, X.; Lu, Q.; Dong, C. The performance of nickel-loaded lignite residue for steam reforming of toluene as the model compound of biomass gasification tar. J. Energy Inst. 2018, 91, 867–876. [Google Scholar] [CrossRef] [Green Version]
- Franz, R. Dry reforming of methane to test passivation stability of Ni/Al2O3 catalysts. Appl. Catal. A Gen. 2021, 7, 117987. [Google Scholar] [CrossRef]
- Cinti, G.; Desideri, U. SOFC fuelled with reformed urea. Appl. Energy 2015, 154, 242–253. [Google Scholar] [CrossRef]
- Fierro, V.; Akdim, O.; Mirodatos, C. On-board hydrogen production in a hybrid electric vehicle by bio-ethanol oxidative steam reforming over Ni and noble metal based catalysts. Green Chem. 2003, 5, 20–24. [Google Scholar] [CrossRef]
- Ruivo, L.; Pio, D.; Yaremchenko, A.; Tarelho, L.; Frade, J.; Kantarelis, E.; Engvall, K. Iron-based catalyst (Fe2-xNixTiO5) for tar decomposition in biomass gasification. Fuel 2021, 300, 120859. [Google Scholar] [CrossRef]
- Jampa, S.; Jamieson, A.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. Achievement of hydrogen production from autothermal steam reforming of methanol over Cu-loaded mesoporous CeO2 and Cu-loaded mesoporous CeO2–ZrO2 catalysts. Int. J. Hydrog. Energy 2017, 42, 15073–15084. [Google Scholar] [CrossRef]
- Pu, J.; Nishikado, K.; Wang, N.; Nguyen, T.; Maki, T.; Qian, E. Core-shell nickel catalysts for the steam reforming of acetic acid. Appl. Catal. B Environ. 2018, 224, 69–79. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Ji, W.-R.; Lempe, D. Density improvement of the SRK equation of state. Fluid Phase Equilibria 1997, 130, 49–63. [Google Scholar] [CrossRef]
- Shatynski, T.; Knox, D. A new density-dependent mixing rule for the SRK equation of state. J Solut. Chem. 1987, 16, 641–648. [Google Scholar] [CrossRef]
- Nikoo, M.; Amin, N. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Ashok, J.; Kathiraser, Y.; Ang, M.; Kawi, S. Bi-functional hydrotalcite-derived NiO–CaO–Al2O3 catalysts for steam reforming of biomass and/or tar model compound at low steam-to-carbon conditions. Appl. Catal. B Environ. 2015, 172–173, 116–128. [Google Scholar] [CrossRef]
- Dang, C.; Liu, L.; Yang, G.; Cai, W.; Long, J.; Yu, H. Mg-promoted Ni-CaO microsphere as bi-functional catalyst for hydrogen production from sorption-enhanced steam reforming of glycerol. Chem. Eng. J. 2020, 383, 123204. [Google Scholar] [CrossRef]
- Amoyal, M.; Vidruk-Nehemya, R.; Landau, M.; Herskowitz, M. Effect of potassium on the active phases of Fe catalysts for carbon dioxide conversion to liquid fuels through hydrogenation. J. Catal. 2017, 348, 29–39. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Schmidt, L. Role of Potassium and Phosphorus in Catalytic Partial Oxidation in Short Contact Time Reactors. Energy Fuels 2019, 29, 8102–8109. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, J.; Du, Y.; Yao, P.; Wang, J.; Lu, J.; Chen, Y. Hydrogen-Rich Syngas Production by Toluene Reforming in a Microchannel Reactor Coated with Ni/MgO–Al 2 O 3 Multifunctional Catalysts. Ind. Eng. Chem. Res. 2019, 58, 19794–19802. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, C.; Ou, Z.; Ran, J. Resistance of Ni/perovskite catalysts to H2S in toluene steam reforming for H2 production. Int. J. Hydrog. Energy 2020, 45, 26800–26811. [Google Scholar] [CrossRef]
- Gao, X.; Ashok, J.; Kawi, S.; Yang, N. Steam reforming of toluene as model compound of biomass tar over Ni–Co/La2O3 nano-catalysts: Synergy of Ni and Co. Int. J. Hydrog. Energy 2021, 46, 30926–30936. [Google Scholar] [CrossRef]
- Virginie, M.; Courson, C.; Kiennemann, A. Toluene steam reforming as tar model molecule produced during biomass gasification with an iron/olivine catalyst. Comptes Rendus Chim. 2010, 13, 1319–1325. [Google Scholar] [CrossRef]
- Soongprasit, K. Synthesis and catalytic activity of sol-gel derived La-Ce-Ni perovskite mixed oxide on steam reforming of toluene. Curr. Appl. Phys. 2012, 12, 9. [Google Scholar] [CrossRef]
- Balonek, C.; Colby, J.; Schmidt, L. Millisecond catalytic reforming of monoaromatics over noble metals. AIChE J. 2009, 56, 979–988. [Google Scholar] [CrossRef]
- Meißner, J.; Pasel, J.; Peters, R.; Samsun, R.; Tschauder, A.; Stolten, D. Elimination of by-products of autothermal diesel reforming. Chem. Eng. J. 2016, 306, 107–116. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.; Li, L.; Zhang, L.; Zhang, J. Properties of methane autothermal reforming to generate hydrogen in membrane reactor based on thermodynamic equilibrium model. Chem. Eng. Process. Process Intensif. 2018, 125, 311–317. [Google Scholar] [CrossRef]









| Catalyst | Temperature (°C) | Conversion (%) | H2-Yield (%) | Reference |
|---|---|---|---|---|
| Ni/MgO-Al2O3 | 750 | 93.0% | 32% | [49] |
| Ni/La0.7Sr0.3AlO3 | 650 | 95.0% | 67% | [50] |
| Ni/LaAlO3 | 600 | 81.0% | 60% | [51] |
| Fe/olivine | 825 | 68.0% | 41% | [52] |
| La0.6Ce0.4NiO3 | 800 | 87% | 79.6% | [53] |
| NiO/K2O-γ-Al2O3 | 700 | >99.9% | 78.9% | This work |
| Number | Reactants | C wt% | H wt% |
|---|---|---|---|
| 1 | C9 | 90.75 | 9.25 |
| 2 | C10 | 94.905 | 5.095 |
| 3 | Diesel | 86.26 | 13.24 |
| 4 | Kerosene | 85.52 | 14.18 |
| 5 | Raffinate oil | 83.365 | 16.635 |
| Molecular Formula | Molecular Weight | Low Heating Value kJ/mol | Standard Enthalpy kJ/mol |
|---|---|---|---|
| C7H8 | 92 | 3905 | |
| H2 | 2 | 2418.260 | |
| H2O(L) | 18 | 285.830 | |
| O2 | 32 | ||
| CH4 | 16 | 890.8 | |
| CO2 | 44 | 393.522 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, C.; Huang, J.; Zhong, B.; Zheng, Z.; Dang, D.; Okafor, O.C.; Liu, Y.; Wang, T. Autothermal Reforming of Volatile Organic Compounds to Hydrogen-Rich Gas. Molecules 2023, 28, 752. https://doi.org/10.3390/molecules28020752
Bian C, Huang J, Zhong B, Zheng Z, Dang D, Okafor OC, Liu Y, Wang T. Autothermal Reforming of Volatile Organic Compounds to Hydrogen-Rich Gas. Molecules. 2023; 28(2):752. https://doi.org/10.3390/molecules28020752
Chicago/Turabian StyleBian, Chao, Jiazhun Huang, Biqi Zhong, Zefeng Zheng, Dai Dang, Obiefuna C. Okafor, Yujia Liu, and Tiejun Wang. 2023. "Autothermal Reforming of Volatile Organic Compounds to Hydrogen-Rich Gas" Molecules 28, no. 2: 752. https://doi.org/10.3390/molecules28020752