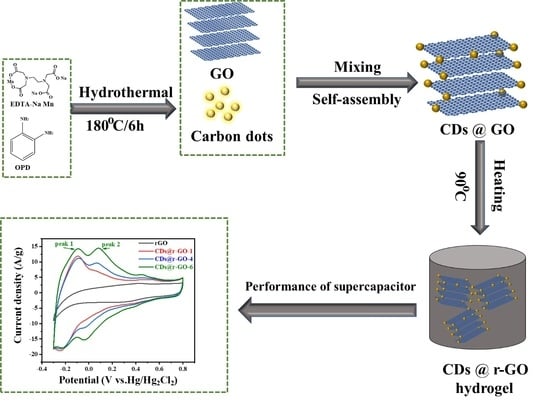
Preparation of Carbon Dots@r-GO Nanocomposite with an Enhanced Pseudo-Capacitance
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Carbon Dots (CDs)
3.3. Preparation of r-GO
3.4. Preparation of CDs @ r-GO Composite Material
3.5. Characterization
3.6. Assembly of Supercapacitor Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wei, Y.; Zheng, M.; Luo, W.; Dai, B.; Ren, J.; Ma, M.; Li, T.; Ma, Y. All pseudocapacitive MXene-MnO2 flexible asymmetric supercapacitor. J. Energy Storage 2022, 45, 103715. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Anjum, D.; Alshareef, H.N. Direct Chemical Synthesis of MnO2 Nanowhiskers on Transition-Metal Carbide Surfaces for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2016, 8, 18806–18814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-Y.; Chang, K.-H.; Tien, H.-W.; Lee, Y.-F.; Li, S.-M.; Wang, Y.-S.; Wang, J.-Y.; Ma, C.-C.M.; Hu, C.-C. Design and tailoring of a hierarchical graphene-carbon nanotube architecture for supercapacitors. J. Mater. Chem. 2011, 21, 2374–2380. [Google Scholar] [CrossRef]
- Fan, W.; Xia, Y.-Y.; Tjiu, W.W.; Pallathadka, P.K.; He, C.; Liu, T. Nitrogen-doped graphene hollow nanospheres as novel electrode materials for supercapacitor applications. J. Power Sources 2013, 243, 973–981. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, N.; Kim, B.G.; Jung, D.S.; Im, K.; Hur, J.; Choi, J.W. Restacking-inhibited 3D reduced graphene oxide for high performance supercapacitor electrodes. ACS Nano 2013, 7, 9366–9374. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, F.-G.; Liu, L.-N.; Xu, Z.-W.; Xie, G.; Li, J.; Gao, T.; Li, W.; Li, W.-S. Carbon Nanomaterials-Enabled High-Performance Supercapacitors: A Review. Adv. Energy Sustain. Res. 2022, 2200152. [Google Scholar] [CrossRef]
- Shi, J.-L.; Du, W.-C.; Yin, Y.-X.; Guo, Y.-G.; Wan, L.-J. Hydrothermal reduction of three-dimensional graphene oxide for binder-free flexible supercapacitors. J. Mater. Chem. A 2014, 2, 10830–10834. [Google Scholar] [CrossRef]
- Sheng, K.-X.; Xu, Y.-X.; Chun, L.; Shi, G.-Q. High-performance self-assembled graphene hydrogels prepared by chemical reduction of graphene oxide. New Carbon Mater. 2011, 26, 9–15. [Google Scholar] [CrossRef]
- Li, J.; Yun, X.; Hu, Z.; Xi, L.; Li, N.; Tang, H.; Lu, P.; Zhu, Y. Three-dimensional nitrogen and phosphorus co-doped carbon quantum dots/reduced graphene oxide composite aerogels with a hierarchical porous structure as superior electrode materials for supercapacitors. J. Mater. Chem. A 2019, 7, 26311–26325. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Yun, X.; Chen, X.; Yao, J.; Lu, Z. Design and fabrication of advanced cathode and anode materials for hybrid supercapacitors based on graphitic carbon quantum dot-decorated reduced graphene oxide composite aerogels. ACS Appl. Energy Mater. 2021, 4, 714–729. [Google Scholar] [CrossRef]
- Zou, Y.; Zhong, W.; Li, S.; Luo, J.; Xiong, C.; Yang, W. Structure of functionalized nitrogen-doped graphene hydrogels derived from isomers of phenylenediamine and graphene oxide based on their high electrochemical performance. Electrochim. Acta 2016, 212, 828–838. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Huang, X.; Wang, Y.; Huang, Y.; Duan, X. Functionalized graphene hydrogel-based high-performance supercapacitors. Adv. Mater. 2013, 25, 5779–5784. [Google Scholar] [CrossRef]
- Wei, J.-S.; Song, T.-B.; Zhang, P.; Niu, X.-Q.; Chen, X.-B.; Xiong, H.-M. A new generation of energy storage electrode materials constructed from carbon dots. Mater. Chem. Front. 2020, 4, 729–749. [Google Scholar] [CrossRef]
- Wei, J.-S.; Ding, C.; Zhang, P.; Ding, H.; Niu, X.-Q.; Ma, Y.-Y.; Li, C.; Wang, Y.-G.; Xiong, H.-M. Robust Negative Electrode Materials Derived from Carbon Dots and Porous Hydrogels for High-Performance Hybrid Supercapacitors. Adv. Mater. 2019, 31, 1806197. [Google Scholar] [CrossRef]
- Rasal, A.S.; Yadav, S.; Yadav, A.; Kashale, A.A.; Manjunatha, S.T.; Altaee, A.; Chang, J.-Y. Carbon Quantum Dots for Energy Applications: A Review. ACS Appl. Nano Mater. 2021, 4, 6515–6541. [Google Scholar] [CrossRef]
- Xiao, J.; Momen, R.; Liu, C. Application of carbon quantum dots in supercapacitors: A mini review. Electrochem. Commun. 2021, 132, 107143. [Google Scholar] [CrossRef]
- Khan, F.; Oh, M.; Kim, J.H. N-functionalized graphene quantum dots: Charge transporting layer for high-rate and durable Li4Ti5O12-based Li-ion battery. Chem. Eng. J. 2019, 369, 1024–1033. [Google Scholar] [CrossRef]
- Jin, J.-C.; Xu, Z.-Q.; Dong, P.; Lai, L.; Lan, J.-Y.; Jiang, F.-L.; Liu, Y. One-step synthesis of silver nanoparticles using carbon dots as reducing and stabilizing agents and their antibacterial mechanisms. Carbon 2015, 94, 129–141. [Google Scholar] [CrossRef]
- Shen, L.; Chen, M.; Hu, L.; Chen, X.; Wang, J. Growth and stabilization of silver nanoparticles on carbon dots and sensing application. Langmuir 2013, 29, 16135–16140. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Wang, J.; Lv, Q.; Jiao, Y.; Li, J.; Li, W.; Akpinar, I.; Shen, W.; He, G. Interfacial engineering of reduced graphene oxide for high-performance supercapacitor materials. J. Electroanal. Chem. 2020, 878, 114679. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gomes, V.G. High efficiency supercapacitor derived from biomass based carbon dots and reduced graphene oxide composite. J. Electroanal. Chem. 2019, 832, 87–96. [Google Scholar]
- Xu, J.; Liang, Q.; Li, Z.; Osipov, V.Y.; Lin, Y.; Ge, B.; Xu, Q.; Zhu, J.; Bi, H. Rational Synthesis of Solid-State Ultraviolet B Emitting Carbon Dots via Acetic Acid-Promoted Fractions of sp3 Bonding Strategy. Adv. Mater. 2022, 34, 2200011. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.K.; Thakur, M.; Bahadur, R.; Kaku, T.; Prabhuraj, R.; Ninawe, A.; Srivastava, R. Preparation of graphene oxide-graphene quantum dots hybrid and its application in cancer theranostics. Mater. Sci. Eng. C 2019, 103, 109774. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Liu, J.; Wu, J.; Chen, H.; Bi, H. Design and preparation of a ternary composite of graphene oxide/carbon dots/polypyrrole for supercapacitor application: Importance and unique role of carbon dots. Carbon 2017, 115, 134–146. [Google Scholar] [CrossRef]
- Yuan, G.; Zhao, X.; Liang, Y.; Peng, L.; Dong, H.; Xiao, Y.; Hu, C.; Hu, H.; Liu, Y.; Zheng, M. Small nitrogen-doped carbon dots as efficient nanoenhancer for boosting the electrochemical performance of three-dimensional graphene. J. Colloid Interface Sci. 2019, 536, 628–637. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L.; Bangal, P.R. Chemical Reduction of Graphene Oxide to Graphene by Sulfur-Containing Compounds. J. Phys. Chem. C 2010, 114, 19885–19890. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Wei, J.-S.; Song, T.-B.; Zhang, P.; Zhu, Z.-Y.; Dong, X.-Y.; Niu, X.-Q.; Xiong, H.-M. Integrating Carbon Dots with Porous Hydrogels to Produce Full Carbon Electrodes for Electric Double-Layer Capacitors. ACS Appl. Energy Mater. 2020, 3, 6907–6914. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Mohan, V.B.; Brown, R.; Jayaraman, K.; Bhattacharyya, D. Characterisation of reduced graphene oxide: Effects of reduction variables on electrical conductivity. Mater. Sci. Eng. B 2015, 193, 49–60. [Google Scholar] [CrossRef]
- Zhou, L.; Song, F.; Yi, J.; Xu, T.; Chen, Q. Nitrogen–Oxygen Co-Doped Carbon-Coated Porous Silica/Carbon Nanotube Composites: Implications for High-Performance Capacitors. ACS Appl. Nano Mater. 2022, 5, 2175–2186. [Google Scholar] [CrossRef]
- Chen, B.; Wu, W.; Li, C.; Wang, Y.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhang, L.; Wu, Y. Oxygen/phosphorus co-doped porous carbon from cicada slough as high-performance electrode material for supercapacitors. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Song, Y.; Ye, Y.-J.; Zhang, M.; Sun, X.; Liu, X.-X. Boosting the pseudocapacitance of nitrogen-rich carbon nanorod arrays for electrochemical capacitors. J. Mater. Chem. A 2019, 7, 12086–12094. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; An, N.; Wang, X.; Yang, Y.; Hu, Z. Covalently functionalized heterostructured carbon by redox-active p-phenylenediamine molecules for high-performance symmetric supercapacitors. New J. Chem. 2019, 43, 1688–1698. [Google Scholar] [CrossRef]
- Yu, X.; Pei, C.; Feng, L. Surface modulated hierarchical graphene film via sulfur and phosphorus dual-doping for high performance flexible supercapacitors. Chin. Chem. Lett. 2019, 30, 1121–1125. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Śliwak, A.; Grzyb, B.; Díez, N.; Gryglewicz, G. Nitrogen-doped reduced graphene oxide as electrode material for high rate supercapacitors. Appl. Surf. Sci. 2017, 399, 265–271. [Google Scholar] [CrossRef]
- Tian, K.; Wang, J.; Cao, L.; Yang, W.; Guo, W.; Liu, S.; Li, W.; Wang, F.; Li, X.; Xu, Z.; et al. Single-site pyrrolic-nitrogen-doped sp2-hybridized carbon materials and their pseudocapacitance. Nat. Commun. 2020, 11, 3884. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Chang, K.-H.; Hu, C.-C. Differentiate the pseudocapacitance and double-layer capacitance contributions for nitrogen-doped reduced graphene oxide in acidic and alkaline electrolytes. J. Power Sources 2013, 227, 300–308. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Wang, H.; Ding, J.; Zahiri, B.; Holt, C.M.B.; Tan, X.; Mitlin, D. Colossal pseudocapacitance in a high functionality–high surface area carbon anode doubles the energy of an asymmetric supercapacitor. Energy Environ. Sci. 2014, 7, 1708–1718. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Tan, J.; Wu, Y.; Liu, S. Hydrothermal carbonization of carboxymethylcellulose: One-pot preparation of conductive carbon microspheres and water-soluble fluorescent carbon nanodots. Chem. Eng. J. 2015, 266, 112–120. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Zhang, Y.; Feng, T.; Wang, J.; Mao, S.; Xiong, L. Porous and high electronic conductivity nitrogen-doped nano-sheet carbon derived from polypyrrole for high-power supercapacitors. Carbon 2016, 107, 638–645. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, T.; Fang, J.; Shi, L.; Zhang, D. Nitrogen-doped porous carbon derived from a bimetallic metal–organic framework as highly efficient electrodes for flow-through deionization capacitors. J. Mater. Chem. A 2016, 4, 10858–10868. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, Z. Recent breakthroughs in supercapacitors boosted by nitrogen-rich porous carbon materials. Adv. Sci. 2017, 4, 1600408. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.-S.; Chen, J.; Ding, H.; Zhang, P.; Wang, Y.-G.; Xiong, H.-M. High volumetric supercapacitor with a long life span based on polymer dots and graphene sheets. J. Power Sources 2017, 364, 465–472. [Google Scholar] [CrossRef]




| Samples | C (wt.%) | O (wt.%) | N (wt.%) |
|---|---|---|---|
| GO | 73.0 | 24.4 | 2.6 |
| CDs@r-GO-1 | 80.2 | 15.5 | 4.3 |
| CDs@r-GO-4 | 85.1 | 9.1 | 5.8 |
| CDs@r-GO-6 | 86.2 | 5.3 | 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Ge, K.; Wu, X.; Zhu, Z.; Zhu, Y.; Bi, H. Preparation of Carbon Dots@r-GO Nanocomposite with an Enhanced Pseudo-Capacitance. Molecules 2023, 28, 541. https://doi.org/10.3390/molecules28020541
Liu Q, Ge K, Wu X, Zhu Z, Zhu Y, Bi H. Preparation of Carbon Dots@r-GO Nanocomposite with an Enhanced Pseudo-Capacitance. Molecules. 2023; 28(2):541. https://doi.org/10.3390/molecules28020541
Chicago/Turabian StyleLiu, Qichen, Kangkang Ge, Xiaoyan Wu, Zhiwei Zhu, Yu Zhu, and Hong Bi. 2023. "Preparation of Carbon Dots@r-GO Nanocomposite with an Enhanced Pseudo-Capacitance" Molecules 28, no. 2: 541. https://doi.org/10.3390/molecules28020541