Glochodpurnoid B from Glochidion puberum Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
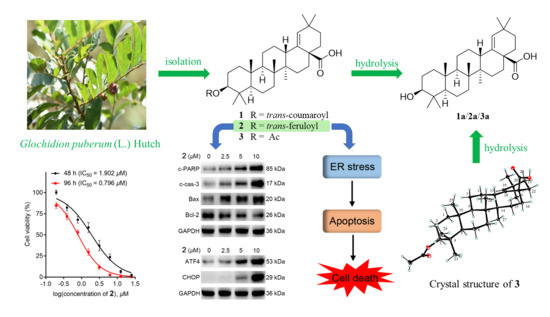
2.1. Isolation and Structure Elucidation
2.2. 2 Inhibited Cell Proliferation in Colorectal Cancer (CRC) Cell Line HCT-116
2.3. 2 Induced Apoptosis in HCT-116 Cells
2.4. 2 Stimulated Endoplasmic Reticulum (ER) Stress-Mediated Apoptosis in HCT-116 Cells
2.5. 2 Potentiated the Antitumor Activity of 5-FU in HCT-116 Cells
3. Materials and Methods
3.1. Plant Material
3.2. General Experimental Procedures
3.3. Extraction and Isolation
3.4. Glochidpurnoid A (1)
3.5. Glochidpurnoid B (2)
3.6. X-ray Crystal Structure Analysis of 3
3.7. Alkaline Hydrolysis of 1–3
3.8. Cell Culture
3.9. Cell Viability Assay
3.10. Colony Formation Assay
3.11. Western Blot Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Md, S.H.; Hidayah, K.; Ammar, A.J.; Zannat, U.; Der, J.O.; Akbar, J.; Ya, C.L.; Kaderi Kibria, K.M.; Mohiuddin, A.K.M.; Long, C.M.; et al. Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers 2022, 14, 1732. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.M.; Stojanovska, V.; Bornstein, J.C.; Nurgal, K. Colorectal cancer chemotherapy: The evolution of treatment and new approaches. Curr. Med. Chem. 2017, 24, 1537–1557. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-Fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2019, 206, 107447. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Zhang, H.; Ma, J.Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef]
- Huang, J.L.; Yan, X.L.; Li, W.; Fan, R.Z.; Li, S.; Chen, J.H.; Zhang, Z.H.; Sang, J.; Gan, L.; Tang, G.H.; et al. Discovery of highly potent daphnane diterpenoids uncovers importin-β1 as a druggable vulnerability in castration-resistant prostate cancer. J. Am. Chem. Soc. 2022, 144, 17522–17532. [Google Scholar] [CrossRef]
- Yao, G.; Li, Y.L.; Luo, S.X. New records of species from several provinces in China. Acta. Bot. Boreal. Occident. Sin. 2019, 39, 1122–1124. [Google Scholar]
- Huang, A.J. An experimental study on eliminating inflammation and analgesic effect of the extract of Glochidion Puberum. J. Hubei Univ. Natl. (Med. Ed.) 2010, 27, 17–19. [Google Scholar]
- Zhang, Z.; Xiao, H.; Liu, G.M. Advances in the research on Glochidion. Chin. J. Ethnomed. Ethnopharm. 2012, 22, 50–51. [Google Scholar]
- Tanaka, R.; Kinouchi, Y.; Wada, S.I.; Tokuda, H. Potential anti-tumor promoting activity of lupane-type triterpenoids from the stem bark of Glochidion zeylanicum and Phyllanthus flexuosus. Planta Med. 2004, 70, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Puapairoj, P.; Naengchomnong, W.; Kijjoa, A.; Pinto, M.M.; Pedro, M.; Nascimento, M.S.J.; Silva, A.M.S.; Herz, W. Cytotoxic activity of lupane-type triterpenes from Glochidion sphaerogynum and Glochidion eriocarpum two of which induce apoptosis. Planta Med. 2005, 71, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.Y.; Xu, F.; Fan, R.Z.; Li, W.; Huang, D.; Tang, G.H.; Yuan, T.; Gan, L.S.; Yin, S. Structural elucidation of three 9,11-seco tetracyclic triterpenoids enables the structural revision of euphorol J. J. Org. Chem. 2021, 86, 7588–7593. [Google Scholar] [CrossRef]
- Li, W.; Lou, L.L.; Zhu, J.Y.; Zhang, J.S.; Liang, A.A.; Bao, J.M.; Tang, G.H.; Yin, S. New lanostane-type triterpenoids from the fruiting body of Ganoderma hainanense. Fitoterapia 2016, 115, 24–30. [Google Scholar] [CrossRef]
- Luo, S.Y.; Pu, R.; Tang, Y.Q.; Fan, R.Z.; Yin, S.; Tang, G.H. Euphane- and 19(10→9)abeo-euphane-type triterpenoids from Jatropha gossypiifolia. Fitoterapia 2020, 143, 104582. [Google Scholar] [CrossRef]
- Chien, N.Q.; Hung, N.V.; Santarsiero, B.D.; Mesecar, A.D.; Cuong, N.M.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.H.S.; Tan, G.T. New 3-O-acyl betulinic acids from Strychnos vanprukii Craib. J. Nat. Prod. 2004, 67, 994–998. [Google Scholar] [CrossRef]
- Zhang, P.; Hao, J.; Liu, J.; Zhang, L.Y.; Sun, H.B. Efficient synthesis of morolic acid and related triterpenes starting from botulin. Tetrahedron 2009, 65, 4304–4309. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Fraga, B.M.; Gonzalez, P.; Hernandez, M.G.; Ravelo, A.G. 3C NMR spectra of olean-18-ene derivatives. Phytochemistry 1981, 20, 1919–1921. [Google Scholar] [CrossRef]
- Fortie, J.; Bohle, D.S.; Leimanis, M.L.; Georges, E.; Rukunga, G.; Nkengfack, A.E. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda. J. Nat. Prod. 2006, 69, 62–67. [Google Scholar]
- Tinto, W.F.; Blair, L.C.; Alli, A. Lupane triteroenoids of Salacza cordata. J. Nat. Prod. 1992, 55, 385–398. [Google Scholar] [CrossRef]
- Wang, X.C.; Ouyang, X.W.; Hu, L.H. Three new lupane-type triterpenes from Ceriops tagal. J. Asian Nat. Prod. Res. 2010, 12, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Hwanga, B.Y.; Chaia, H.B.; Kardonob, L.B.S.; Riswanc, S.; Farnswortha, N.R.; Cordella, G.A.; Pezzutoa, J.M.; Kinghorna, A.D. Cytotoxic triterpenes from the twigs of Celtis philippinensis. Phytochemistry 2003, 62, 197–201. [Google Scholar] [CrossRef]
- Pakhathirathien, C.; Karalai, C.; Ponglimanont, C.; Subhadhirasakul, S.; Chantrapromma, K. Dammarane triterpenes from the Hypocotyls and fruits of Ceriops tagal. J. Nat. Prod. 2005, 68, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Al Musayeib, N.M.; Mothana, R.A.; El Gamal, A.A.; Al-Massarani, S.M.; Maes, L. In vitro antiprotozoal activity of triterpenoid constituents of Kleinia odora growing in Saudi Arabia. Molecules 2013, 18, 9207–9218. [Google Scholar] [CrossRef]
- Ullah, N.; Ahmed, Z.; Ahmed, S.; Muhammad, P.; Malik, A. A pentacyclic triterpene from Daphne oleoides. Phytochemistry 1999, 50, 839–841. [Google Scholar] [CrossRef]
- Carpenter, R.C.; Sotheeswaran, S.; Sultanbawa, M.U.S.; Balasubramaniam, S. Triterpenes of five Euphorbiaceae species of Sri lanka. Phytochemistry 1980, 19, 1171–1174. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, H.J.; Tan, G.T.; Hung, N.V.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.S. Antimalarial compounds from Grewia bilamellata. J. Nat. Prod. 2006, 69, 346–350. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.J.; Yang, J.Z.; Ma, J.; Li, Y.; Bao, X.Q.; Chen, X.G.; Zhang, D.; Zhang, D.M. Lupane triterpenoids from the stems of Euonymus carnosus. J. Nat. Prod. 2014, 77, 276–284. [Google Scholar] [CrossRef]
- Sichaem, J.; Vo, H.C.; Nha-Tran, T.; Jarupinthusophon, S.; Niamnont, N.; Srikittiwanna, K.; Nguyen, T.K.; Tran, T.; Le, T.; Duong, T. 29-Norlupane-1β-hydroxy-3,20-dione, a new norlupane triterpenoid from the twigs and leaves of Phyllanthus acidus. Nat. Prod. Res. 2021, 35, 3384–3389. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.P.; Nunez, M.J.; Jimenez, I.A.; Busserolles, J.; Alcarazb, M.J.; Bazzocchia, I.L. Activity of lupane triterpenoids from Maytenus species as inhibitors of nitric oxide and prostaglandin E2. Bioorg. Med. Chem. 2006, 14, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Morikawa, T.; Oominami, H.; Matsuda, H. Absolute stereostructures of olibanumols A, B, C, H, I, and J from olibanum, gum-Resin of Boswellia carterii, and inhibitors of nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages. Chem. Pharm. Bull. 2009, 57, 957–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thongnest, S.; Boonsombat, J.; Prawat, H.; Mahidol, C.; Ruchirawat, S. Ailanthusins A-G and nor-lupane triterpenoids from Ailanthus triphysa. Phytochemistry 2017, 134, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Claudia, B.; Ioana, B.H.; Ovidiu, B.; Alexandru, I. Apoptosis in cancer: Key molecular signaling pathways and therapy targets. Acta Oncol. 2009, 48, 811–821. [Google Scholar]
- Gou, W.F.; Luo, N.; Yu, B.; Wu, H.Y.; Wu, S.H.; Tian, C.; Guo, J.H.; Ning, H.X.; Bi, C.F.; Wei, H.Q.; et al. Acid derivative UA232 promotes tumor cell apoptosis by inducing endoplasmic reticulum stress and lysosomal dysfunction. Int. J. Biol. Sci. 2022, 18, 2639–2651. [Google Scholar] [CrossRef]
- Zhan, Z.J.; Li, S.; Chu, W.; Yin, S. Euphorbia diterpenoids: Isolation, structure, bioactivity, biosynthesis, and synthesis (2013–2021). Nat. Prod. Rep. 2022, 39, 2132–2174. [Google Scholar] [CrossRef]





| No. | 1 a | 2 b | ||
|---|---|---|---|---|
| δH, Multi. (J in Hz) | δC, Type | δH, Multi. (J in Hz) | δC, Type | |
| 1 | α 0.92, m β 1.65, m | 39.0, CH2 | α 1.06, m β 1.75, m | 38.6, CH2 |
| 2 | α 1.88, m β 1.79, m | 24.6, CH2 | a 1.73, m b 1.73, m | 23.8, CH2 |
| 3 | 4.90, dd (11.6, 4.7) | 80.9, CH | 4.63, dd (10.5, 5.5) | 80.8, CH |
| 4 | 38.6, C | 38.1, C | ||
| 5 | 0.87, m | 56.1, CH | 0.85, d (9.7) | 55.6, CH |
| 6 | α 1.31, m β 1.49, m | 18.8, CH2 | α 1.51, m β 1.37, m | 18.1, CH2 |
| 7 | a 1.43, m b 1.34, m | 35.2, CH2 | a 1.47, m b 1.35, m | 34.5, CH2 |
| 8 | 41.3, C | 40.7, C | ||
| 9 | 1.34, m | 51.7, CH | 1.30, m | 51.1, CH |
| 10 | 37.7, C | 37.1, C | ||
| 11 | a 1.49, m b 1.21, m | 21.7, CH2 | α 1.57, m β 1.28, m | 20.9, CH2 |
| 12 | α 1.33, m β 1.73, m | 26.8, CH2 | a 1.62, m b 1.25, m | 26.0, CH2 |
| 13 | 2.72, d (11.2) | 42.0, CH | 2.22, d (9.7) | 41.3, CH |
| 14 | 43.4, C | 42.5, C | ||
| 15 | α 1.46, m β 2.03, m | 30.3, CH2 | a 1.68, m b 1.23, m | 29.4, CH2 |
| 16 | α 1.33, m β 1.82, m | 34.7, CH2 | a 2.16, m b 1.40, m | 33.5, CH2 |
| 17 | 48.9, C | 47.9, C | ||
| 18 | 139.3, C | 136.7, C | ||
| 19 | 5.33, s | 132.5, CH | 5.17, s | 133.2, CH |
| 20 | 32.8, C | 32.0, C | ||
| 21 | a 1.46, m b 1.46, m | 34.6, CH2 | a 1.99, m b 1.40, m | 33.4, CH2 |
| 22 | a 2.36, m b 2.36, m | 34.6, CH2 | a 2.01, m b 1.65, m | 33.3, CH2 |
| 23 | 0.97, s | 28.5, CH3 | 0.89, s | 28.0, CH3 |
| 24 | 1.04, s | 17.3, CH3 | 0.92, s | 16.7, CH3 |
| 25 | 0.82, s | 17.1, CH3 | 1.00, s | 16.0, CH3 |
| 26 | 0.99, s | 16.6, CH3 | 0.91, s | 16.7, CH3 |
| 27 | 0.99, s | 15.7, CH3 | 0.79, s | 14.9, CH3 |
| 28 | 179.4, C | 182.2, C | ||
| 29 | 1.16, s | 31.2, CH3 | 1.00, s | 30.3, CH3 |
| 30 | 1.10, s | 29.7, CH3 | 0.98, s | 29.1, CH3 |
| 1′ | 167.7, C | 167.2, C | ||
| 2′ | 6.74, d (15.9) | 116.2, CH | 6.29, d (15.9) | 116.2, CH |
| 3′ | 8.07, d (15.9) | 145.4, CH | 7.59, d (15.9) | 144.4, CH |
| 4′ | 126.6, C | 127.1, C | ||
| 5′ | 7.69, d (8.6) | 131.1, CH | 7.03, d (1.7) | 109.3, CH |
| 6′ | 7.20, m | 117.3, CH | 146.7, C | |
| 7′ | 161.8, C | 147.8, C | ||
| 8′ | 7.20, m | 117.3, CH | 6.91, d (8.2) | 114.7, CH |
| 9′ | 7.69, d (8.6) | 131.1, CH | 7.07, dd (8.2, 1.7) | 123.0, CH |
| 6′-OCH3 | 3.93, s | 55.9, CH3 | ||
| Compound | IC50 (μM) | Compound | IC50 (μM) | ||
|---|---|---|---|---|---|
| 48 h | 96 h | 48 h | 96 h | ||
| 2 | 1.90 ± 0.02 | 0.80 ± 0.05 | 11 | 5.30 ± 0.59 | 2.91 ± 0.06 |
| 3 | 7.12 ± 0.60 | 2.99 ± 0.34 | 17 | 5.33 ± 0.18 | 2.40 ± 0.47 |
| 5 | 2.75 ± 0.05 | 1.89 ± 0.02 | 5-FU b | 13.50 ± 0.59 | 3.79 ± 0.17 |
| 6 | 2.40 ± 0.71 | 1.16 ± 0.07 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Fan, R.; Yin, Z.; Huang, Y.; Huang, D.; Yuan, F.; Yin, A.; Tang, G.; Pu, R.; Yin, S. Glochodpurnoid B from Glochidion puberum Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Colorectal Cancer Cells. Molecules 2023, 28, 511. https://doi.org/10.3390/molecules28020511
Tian Y, Fan R, Yin Z, Huang Y, Huang D, Yuan F, Yin A, Tang G, Pu R, Yin S. Glochodpurnoid B from Glochidion puberum Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Colorectal Cancer Cells. Molecules. 2023; 28(2):511. https://doi.org/10.3390/molecules28020511
Chicago/Turabian StyleTian, Yang, Runzhu Fan, Zhao Yin, Yongping Huang, Dong Huang, Fangyu Yuan, Aiping Yin, Guihua Tang, Rong Pu, and Sheng Yin. 2023. "Glochodpurnoid B from Glochidion puberum Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Colorectal Cancer Cells" Molecules 28, no. 2: 511. https://doi.org/10.3390/molecules28020511