Investigation of the Vibrational Characteristics of 6-Isocyano-1-Methyl-1H-Indole: Utilizing the Isonitrile Group as an Infrared Probe
Abstract
:1. Introduction
2. Results and Discussion
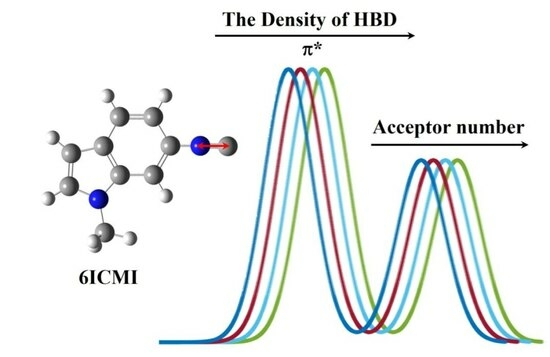
2.1. FTIR Spectroscopy
2.2. Quantum Chemical Calculation
2.3. Polarization-Controlled IR Pump-Probe Spectroscopy
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Spectroscopic Measurements
3.3. Computational
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gummidi, L.; Kerru, N.; Awolade, P.; Raza, A.; Sharma, A.K.; Singh, P. Synthesis of indole-tethered [1, 3, 4] thiadiazolo and [1, 3, 4] oxadiazolo [3, 2-a] pyrimidin-5-one hybrids as anti-pancreatic cancer agents. Bioorg. Med. Chem. Lett. 2020, 30, 127544. [Google Scholar] [CrossRef]
- Siddiqui, S.K.; SahayaSheela, V.J.; Kolluru, S.; Pandian, G.N.; Santhoshkumar, T.R.; Dan, V.M.; Ramana, C.V. Discovery of 3-(benzofuran-2-ylmethyl)-1H-indole derivatives as potential autophagy inducers in cervical cancer cells. Bioorg. Med. Chem. Lett. 2020, 30, 127431. [Google Scholar] [CrossRef]
- Kulkarni, A.; Soni, I.; Kelkar, D.S.; Dharmaraja, A.T.; Sankar, R.K.; Beniwal, G.; Rajendran, A.; Tamhankar, S.; Chopra, S.; Kamat, S.S. Chemoproteomics of an indole-based quinone epoxide identifies druggable vulnerabilities in vancomycin-resistant Staphylococcus aureus. J. Med. Chem. 2019, 62, 6785–6795. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Ragab, A.; Askar, A.A.; El-Khalafawy, A.; Makhlouf, A.H. One-pot synthesis and molecular docking of some new spiropyranindol-2-one derivatives as immunomodulatory agents and in vitro antimicrobial potential with DNA gyrase inhibitor. Eur. J. Med. Chem. 2020, 188, 111977. [Google Scholar] [CrossRef]
- Martinez-Gualda, B.; Sun, L.; Martí-Marí, O.; Noppen, S.; Abdelnabi, R.; BaEtor, C.M.; Quesada, E.; Delang, L.; Mirabelli, C.; Lee, H. Scaffold simplification strategy leads to a novel generation of dual human immunodeficiency virus and enterovirus-A71 entry inhibitors. J. Med. Chem. 2019, 63, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chu, S.; Nemati, A.; Huang, C.; Snyder, B.A.; Ptak, R.G.; Gochin, M. Investigation of the molecular characteristics of bisindole inhibitors as HIV-1 glycoprotein-41 fusion inhibitors. Eur. J. Med. Chem. 2019, 161, 533–542. [Google Scholar] [CrossRef] [PubMed]
- ElBordiny, H.S.; El-Miligy, M.M.; Kassab, S.E.; Daabees, H.; Ali, W.A.M.; El-Hawash, S.A.M. Design, synthesis, biological evaluation and docking studies of new 3-(4, 5-dihydro-1H-pyrazol/isoxazol-5-yl)-2-phenyl-1H-indole derivatives as potent antioxidants and 15-lipoxygenase inhibitors. Eur. J. Med. Chem. 2018, 145, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Eeda, V.; Wu, D.; Lim, H.-Y.; Wang, W. Design, synthesis, and evaluation of potent novel peroxisome proliferator-activated receptor γ indole partial agonists. Bioorg. Med. Chem. Lett. 2019, 29, 126664. [Google Scholar] [CrossRef] [PubMed]
- Darwish, K.M.; Salama, I.; Mostafa, S.; Gomaa, M.S.; Khafagy, E.-S.; Helal, M.A. Synthesis, biological evaluation, and molecular docking investigation of benzhydrol-and indole-based dual PPAR-γ/FFAR1 agonists. Bioorg. Med. Chem. Lett. 2018, 28, 1595–1602. [Google Scholar] [CrossRef]
- Hou, S.; Yang, X.; Tong, Y.; Yang, Y.; Chen, Q.; Wan, B.; Wei, R.; Wang, Y.; Zhang, Y.; Kong, B. Structure-based discovery of 1H-indole-2-carboxamide derivatives as potent ASK1 inhibitors for potential treatment of ulcerative colitis. Eur. J. Med. Chem. 2021, 211, 113114. [Google Scholar] [CrossRef]
- Moir, M.; Lane, S.; Lai, F.; Connor, M.; Hibbs, D.E.; Kassiou, M. Strategies to develop selective CB2 receptor agonists from indole carboxamide synthetic cannabinoids. Eur. J. Med. Chem. 2019, 180, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, B.; Li, J.; Liu, H.; Zhang, Y.; Yang, Z.; Liu, W. Design, synthesis, biological evaluation and docking study of novel indole-2-amide as anti-inflammatory agents with dual inhibition of COX and 5-LOX. Eur. J. Med. Chem. 2019, 180, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, G.; Li, H.; Chen, W.; Li, J.; Feng, C.; Gu, Z.; Zhu, F.; Zhang, R.; Li, M. Structure-aided identification and optimization of tetrahydro-isoquinolines as novel PDE4 inhibitors leading to discovery of an effective antipsoriasis agent. J. Med. Chem. 2019, 62, 5579–5593. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Cui, P.; Zang, C.; Li, S. Enantioselective total synthesis, divergent optimization and preliminary biological evaluation of (indole-N-alkyl)-diketopiperazines. Bioorg. Med. Chem. Lett. 2019, 29, 126718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Jiang, Z.-Y.; Zhu, Q.; Zhong, G.-H. Discovery of β-carboline oxadiazole derivatives as fungicidal agents against rice sheath blight. J. Agric. Food Chem. 2018, 66, 9598–9607. [Google Scholar] [CrossRef]
- Talukder, P.; Chen, S.; Roy, B.; Yakovchuk, P.; Spiering, M.M.; Alam, M.P.; Madathil, M.M.; Bhattacharya, C.; Benkovic, S.J.; Hecht, S.M. Cyanotryptophans as Novel Fluorescent Probes for Studying Protein Conformational Changes and DNA-Protein Interaction. Biochemistry 2015, 54, 7457–7469. [Google Scholar] [CrossRef]
- Hilaire, M.R.; Ahmed, I.A.; Lin, C.W.; Jo, H.; DeGrado, W.F.; Gai, F. Blue fluorescent amino acid for biological spectroscopy and microscopy. Proc. Natl. Acad. Sci. USA 2017, 114, 6005–6009. [Google Scholar] [CrossRef] [PubMed]
- Hilaire, M.R.; Mukherjee, D.; Troxler, T.; Gai, F. Solvent Dependence of Cyanoindole Fluorescence Lifetime. Chem. Phys. Lett. 2017, 685, 133–138. [Google Scholar] [CrossRef]
- Markiewicz, B.N.; Mukherjee, D.; Troxler, T.; Gai, F. Utility of 5-cyanotryptophan fluorescence as a sensitive probe of protein hydration. J. Phys. Chem. B 2016, 120, 936–944. [Google Scholar] [CrossRef]
- Zhang, W.; Markiewicz, B.N.; Doerksen, R.S.; Smith, A.B., III; Gai, F. C [triple bond, length as m-dash] N stretching vibration of 5-cyanotryptophan as an infrared probe of protein local environment: What determines its frequency? Phys. Chem. Chem. Phys. 2016, 18, 7027–7034. [Google Scholar] [CrossRef]
- Markiewicz, B.N.; Lemmin, T.; Zhang, W.; Ahmed, I.A.; Jo, H.; Fiorin, G.; Troxler, T.; DeGrado, W.F.; Gai, F. Infrared and fluorescence assessment of the hydration status of the tryptophan gate in the influenza A M2 proton channel. Phys. Chem. Chem. Phys. 2016, 18, 28939–28950. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.M.; Abaskharon, R.M.; Ding, B.; Chen, J.; Zhang, W.; Gai, F. Fermi resonance as a means to determine the hydrogen-bonding status of two infrared probes. Phys. Chem. Chem. Phys. 2017, 19, 16144–16150. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Fan, H.; Su, W.; Chen, X.; Zhang, W. Ester-derivatized indoles as sensitive infrared probes for local environment. Chem. Phys. Lett. 2020, 742, 137139. [Google Scholar] [CrossRef]
- You, M.; Zhou, L.; Huang, X.; Wang, Y.; Zhang, W. Isonitrile-Derivatized Indole as an Infrared Probe for Hydrogen-Bonding Environments. Molecules 2019, 24, 1379. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; You, M.; Ran, G.-L.; Fan, H.-R.; Zhang, W.-K. Ester-Derivatized indoles as fluorescent and infrared probes for hydration environments. Chin. J. Chem. Phys 2018, 31, 477. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters,. pi.*,. alpha., and. beta., and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Dickinson, C.; Taft, R. Linear solvation energy relationships Solvent effects on some fluorescence probes. Chem. Phys. Lett. 1981, 77, 69–72. [Google Scholar] [CrossRef]
- Gutmann, V. Solvent effects on the reactivities of organometallic compounds. Coord. Chem. Rev. 1976, 18, 225–255. [Google Scholar] [CrossRef]
- Bauer, M.E.; Magat, M. Sur la deformation des molécules et leurs spectres de vibration dans les etats condenses. Physica 1938, 5, 718–724. [Google Scholar] [CrossRef]
- Mayer, U.; Gutmann, V.; Gerger, W. The acceptor number—A quantitative empirical parameter for the electrophilic properties of solvents. Monatsh. Chem. 1975, 106, 1235–1257. [Google Scholar] [CrossRef]
- Gutmann, V. Empirical parameters for donor and acceptor properties of solvents. Electrochim. Acta 1976, 21, 661–670. [Google Scholar] [CrossRef]
- Maj, M.; Ahn, C.; Kossowska, D.; Park, K.; Kwak, K.; Han, H.; Cho, M. beta-Isocyanoalanine as an IR probe: Comparison of vibrational dynamics between isonitrile and nitrile-derivatized IR probes. Phys. Chem. Chem. Phys. 2015, 17, 11770–11778. [Google Scholar] [CrossRef]
- You, M.; Liu, L.; Zhang, W. The covalently bound diazo group as an infrared probe for hydrogen bonding environments. Phys. Chem. Chem. Phys. 2017, 19, 19420–19426. [Google Scholar] [CrossRef]
- Wolfshorndl, M.P.; Baskin, R.; Dhawan, I.; Londergan, C.H. Covalently bound azido groups are very specific water sensors, even in hydrogen-bonding environments. J. Phys. Chem. B 2012, 116, 1172–1179. [Google Scholar] [CrossRef]
- Moilanen, D.E.; Piletic, I.R.; Fayer, M.D. Water dynamics in Nafion fuel cell membranes: The effects of confinement and structural changes on the hydrogen bond network. J. Phys. Chem. C 2007, 111, 8884–8891. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]











| Solvent | w0(NC) | w1(NC) a | π* | β | α | ε | AN b | DN | f |
|---|---|---|---|---|---|---|---|---|---|
| n-octanol | 2122.6 | 2139.4 | 0.4 | 0.81 | 0.77 | 10.3 | 32 | 0.431 | |
| acetonitrile | 2128.2 | 0.75 | 0.31 | 0.19 | 37.5 | 18.9 | 14.1 | 0.480 | |
| 1,4-dioxane | 2124.9 | 0.55 | 0.37 | 0 | 2.2 | 10.3 | 14.3 | 0.222 | |
| N,N-Dimethylformamide (DMF) | 2125.0 | 0.88 | 0.69 | 0 | 38.2 | 16 | 26.6 | 0.481 | |
| dimethyl sulfoxide (DMSO) | 2125.2 | 1 | 0.76 | 0 | 47.2 | 19.3 | 29.8 | 0.484 | |
| 2-propanol | 2123.9 | 2139.7 | 0.48 | 0.95 | 0.76 | 20.2 | 33.5 | 36 | 0.464 |
| methanol (MeOH) | 2125.5 | 2141.5 | 0.6 | 0.62 | 0.93 | 33 | 41.3 | 30 | 0.478 |
| tetrahydrofuran (THF) | 2123.8 | 0.58 | 0.55 | 0 | 7.5 | 8 | 20 | 0.406 | |
| n-butanol | 2123.6 | 2140.6 | 0.47 | 0.88 | 0.79 | 17.8 | 36.8 | 29 | 0.459 |
| toluene | 2123.7 | 0.54 | 0.11 | 0 | 2.4 | 0.1 | 0.241 | ||
| n-propanol | 2123.9 | 2140.8 | 0.52 | 0.9 | 0.84 | 20.1 | 37.3 | 19.8 | 0.464 |
| ethanol(EtOH) | 2124.3 | 2140.7 | 0.54 | 0.77 | 0.83 | 24.5 | 37.1 | 32 | 0.470 |
| dichloromethane (DCM) | 2128.1 | 0.82 | 0.1 | 0.13 | 8.9 | 20.4 | 1 | 0.420 |
| Solvent | Esolvation | m | Δm | Cacl.w0(NC) | Δw0(NC) | L(NC) | ΔL(NC) | f |
|---|---|---|---|---|---|---|---|---|
| Vacuum | 5.8551 | 2121.26 | 1.1784 | |||||
| Toluene | −14.0895 | 6.7521 | 0.8970 | 2124.49 | 3.24 | 1.1769 | −0.0014 | 0.241 |
| THF | −22.2480 | 7.3319 | 1.4768 | 2126.51 | 5.26 | 1.1761 | −0.0022 | 0.406 |
| DCM | −22.9351 | 7.3831 | 1.5280 | 2126.67 | 5.41 | 1.1761 | −0.0023 | 0.420 |
| Ethanol | −25.1526 | 7.5509 | 1.6958 | 2127.13 | 5.88 | 1.1759 | −0.0025 | 0.470 |
| MeOH | −25.4537 | 7.5740 | 1.7189 | 2127.19 | 5.93 | 1.1758 | −0.0025 | 0.478 |
| Acetonitrile | −25.5372 | 7.5805 | 1.7254 | 2127.21 | 5.95 | 1.1758 | −0.0025 | 0.480 |
| DMSO | −25.7478 | 7.5967 | 1.7416 | 2127.25 | 5.99 | 1.1758 | −0.0026 | 0.484 |
| Explicit Solvent | Esolvation | Calc. w1(NC) | Exp. w1(NC) | Δw1(NC) | L(NC) | ΔL(NC) | AN | f |
|---|---|---|---|---|---|---|---|---|
| Vacuum | 2122.66 | 1.17836 | ||||||
| 2-propanol | −28.3289 | 2153.88 | 2139.70 | 31.22 | 1.17318 | −0.00518 | 33.5 | 0.464 |
| n-butanol | −29.7850 | 2155.17 | 2140.60 | 32.51 | 1.17300 | −0.00536 | 36.8 | 0.459 |
| Ethanol | −29.9885 | 2155.36 | 2140.70 | 32.70 | 1.17297 | −0.00539 | 37.1 | 0.470 |
| 1-propanol | −29.9892 | 2155.44 | 2140.80 | 32.78 | 1.17297 | −0.00539 | 37.3 | 0.464 |
| MeOH | −30.5860 | 2156.34 | 2141.20 | 33.68 | 1.17283 | −0.00553 | 41.3 | 0.478 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, M.; Gao, Z.; Zhou, L.; Guo, C.; Guo, Q. Investigation of the Vibrational Characteristics of 6-Isocyano-1-Methyl-1H-Indole: Utilizing the Isonitrile Group as an Infrared Probe. Molecules 2023, 28, 6939. https://doi.org/10.3390/molecules28196939
You M, Gao Z, Zhou L, Guo C, Guo Q. Investigation of the Vibrational Characteristics of 6-Isocyano-1-Methyl-1H-Indole: Utilizing the Isonitrile Group as an Infrared Probe. Molecules. 2023; 28(19):6939. https://doi.org/10.3390/molecules28196939
Chicago/Turabian StyleYou, Min, Zilin Gao, Liang Zhou, Changyuan Guo, and Qiang Guo. 2023. "Investigation of the Vibrational Characteristics of 6-Isocyano-1-Methyl-1H-Indole: Utilizing the Isonitrile Group as an Infrared Probe" Molecules 28, no. 19: 6939. https://doi.org/10.3390/molecules28196939