The N-Alkylation of Agelastatin A Modulates Its Chemical Reactivity
Abstract
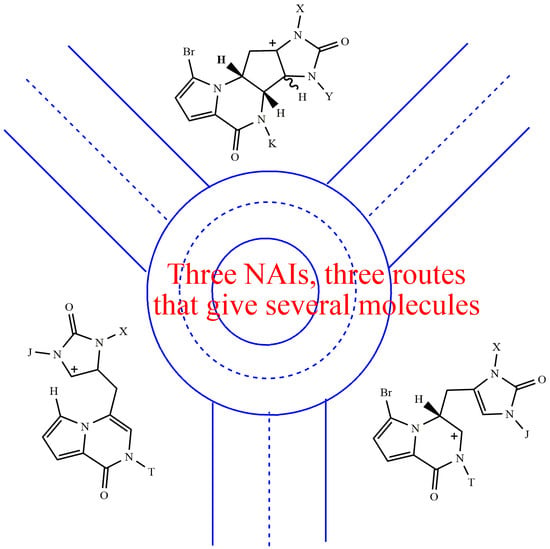
:1. Introduction
2. Results and Discussion
2.1. Reaction Conditions and Products
2.2. Reaction Mechanism
2.3. Deuterium Incorporation: MS and 13C-NMR Studies
2.4. N-Alkylations and Reaction Products
3. Materials and Methods
3.1. Chemicals and Instrumentation
3.2. Reaction Conditions and Products
3.2.1. Rel-(6aR,9aR)-4,7,9-trimethyl-4,6,6a,7,9,9a-hexahydroimidazo [4,5-g]pyrazino [2,1,6-cd]indolizine-3,8-dione (6e)
3.2.2. (5aR,9aR)-1-Bromo-5,6,8-trimethyl-5,5a,6,8,9,9a-hexahydroimidazo [4’,5’:4,5]cyclopenta [1,2-e]pyrrolo [1,2-a]pyrazine-4,7-dione (3e)
3.2.3. 6-Bromo-4-[(2,3-dihydro-1,3-dimethyl-2-oxo-1H-imidazol-4-yl)methyl]-2-methylpyrrolo [1,2-a]pyrazin-1(2H)-one (4e)
3.2.4. 4-[(2,3-Dihydro-1,3-dimethyl-2-oxo-1H-imidazol-4-yl)methyl]-2-methylpyrrolo [1,2-a]pyrazin-1(2H)-one (5e)
3.3. Reaction Mechanism
3.4. Deuterium Incorporation
3.5. N-Alkylations and Reaction Products
3.5.1. (5aS,5bS,8aS,9aR)-1-Bromo-8a-hydroxy-5,8-dimethyl-5,5a,5b,6,8,8a,9,9a-octahydroimidazo [4’,5’:4,5]cyclopenta [1,2-e]pyrrolo [1,2-a]pyrazine-4,7-dione (1d)
3.5.2. (5aS,5bS,8aS,9aR)-1-Bromo-8a-hydroxy-6,8-dimethyl-5,5a,5b,6,8,8a,9,9a-octahydroimidazo [4’,5’:4,5]cyclopenta [1,2-e]pyrrolo [1,2-a]pyrazine-4,7-dione (1m)
3.5.3. (5aS,5bS,8aS,9aR)-1-Bromo-8a-methoxy-5,8-dimethyl-5,5a,5b,6,8,8a,9,9a-octahydroimidazo [4’,5’:4,5]cyclopenta [1,2-e]pyrrolo [1,2-a]pyrazine-4,7-dione (2d)
3.5.4. (5aS,5bS,8aS,9aR)-1-Bromo-8a-methoxy-6,8-dimethyl-5,5a,5b,6,8,8a,9,9a-octahydroimidazo [4’,5’:4,5]cyclopenta [1,2-e]pyrrolo [1,2-a]pyrazine-4,7-dione (2m)
3.5.5. (5aR,9aR)-1-Bromo-6,8-dimethyl-5,5a,6,8,9,9a-hexahydroimidazo [4’,5’:4,5]cyclopenta [1,2-e]pyrrolo [1,2-a]pyrazine-4,7-dione (3m)
3.5.6. (5aR,9aR)-1-Bromo-5,8-dimethyl-5,5a,6,8,9,9a-hexahydroimidazo [4’,5’:4,5]cyclopenta [1,2-e]pyrrolo [1,2-a]pyrazine-4,7-dione (3d)
3.5.7. 4-[(2,3-Dihydro-3-methyl-2-oxo-1H-imidazol-4-yl)methyl]-2-methylpyrrolo [1,2-a]pyrazin-1(2H)-one (5d)
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- D’Ambrosio, M.; Guerriero, A.; Chiasera, G.; Pietra, F. Conformational preferences and absolute configuration of agelastatin A, a cytotoxic alkaloid of the axinellid sponge Agelas dendromorpha from the Coral Sea, via combined molecular modeling, NMR, and exciton splitting for diamide and hydroxyamide derivatives. Helv. Chim. Acta 1994, 77, 1895–1902. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Guerriero, A.; Ripamonti, M.; Debitus, C.; Waikedre, J.; Pietra, F. The active centers of agelastatin A, a strongly cytotoxic alkaloid of the Coral sea axinellid sponge Agelas dendromorpha, as determined by comparative bioassays with semisynthetic derivatives. Helv. Chim. Acta 1996, 79, 727–735. [Google Scholar] [CrossRef]
- Dong, G. Recent advances in the total synthesis of agelastatins. Pure Appl. Chem. 2010, 82, 2231–2314. [Google Scholar] [CrossRef]
- Crossley, S.W.; Shenvi, R.A. A Longitudinal Study of Alkaloid Synthesis Reveals Functional Group Interconversions as Bad Actors. Chem. Rev. 2015, 115, 9465–9531. [Google Scholar] [CrossRef] [PubMed]
- Yoshimitsu, T. Chemical Syntheses and Biological Studies of Agelastatin A, a Bioactive Marine Heterocycle Gifted from Nature. Heterocycles 2020, 100, 1735–1762. [Google Scholar] [CrossRef]
- Wehn, P.M.; Du Bois, J. A stereoselective synthesis of the bromopyrrole natural product (−)-agelastatin A. Angew. Chem. Int. Ed. Engl. 2009, 48, 3802–3805. [Google Scholar] [CrossRef]
- Movassaghi, M.; Siegel, D.S.; Han, S. Total synthesis of all (−)-Agelastatin alkaloids. Chem. Sci. 2010, 1, 561–566. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, X.; Liang, G. Total syntheses of (+)-agelastatin A and (+)-agelastatin B through cationic cyclizations. Tetrahedron 2017, 73, 4538–4544. [Google Scholar] [CrossRef]
- Shigeoka, D.; Kamon, T.; Yoshimitsu, T. Formal synthesis of (−)-agelastatin A: An iron(II)-mediated cyclization strategy. Beilstein J. Org. Chem. 2013, 9, 860–865. [Google Scholar] [CrossRef]
- Reyes, J.C.; Romo, D. Bioinspired Total Synthesis of Agelastatin A. Angew. Chem. Int. Ed. Engl. 2012, 51, 6870–6873. [Google Scholar] [CrossRef]
- Duspara, P.A.; Batey, R.A. A Short Total Synthesis of the Marine Sponge Pyrrole-2-aminoimidazole Alkaloid (±)-Agelastatin A. Angew. Chem. Int. Ed. Engl. 2013, 52, 10862–10866. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.F.A.; Vale, J.R.; Pereira, J.G.; Afonso, C.A.M. Orthogonally Protected Diaminocyclopentenones as Synthons: Total Synthesis of (±)-Agelastatin A. Org. Lett. 2023, 25, 4188–4192. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Siegel, D.S.; Morrison, K.C.; Hergenrother, P.J.; Movassaghi, M. Synthesis and Anticancer Activity of All Known (−)-Agelastatin Alkaloids. J. Org. Chem. 2013, 78, 11970–11984. [Google Scholar] [CrossRef] [PubMed]
- Jouanneau, M.; McClary, B.; Reyes, J.C.P.; Chen, R.; Chen, Y.; Plunkett, W.; Cheng, X.; Milinichik, A.Z.; Albone, E.F.; Liu, J.O.; et al. Derivatization of agelastatin A leading to bioactive analogs and a trifunctional probe. Bioorg. Med. Chem. Lett. 2016, 26, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Svatek, H.; Bertonha, A.F.; Reisenauer, K.; Robinson, J.; Kim, M.; Ingros, A.; Ho, M.; Taube, J.; Romo, D. Synthesis of agelastatin A and derivatives premised on a hidden symmetry element leading to analogs displaying anticancer activity. Tetrahedron 2021, 94, 132340. [Google Scholar] [CrossRef] [PubMed]
- Vale, J.R.; Fortunato, M.A.G.; Andrade, K.H.S.; Rocha, A.M.R.; Afonso, C.A.M.; Siopa, F. From Pyridine to (−)-Agelastatin A. Adv. Synth. Catal. 2023, 365, 2240–2247. [Google Scholar] [CrossRef]
- Li, Z.; Shigeoka, D.; Caulfield, T.R.; Kawachi, T.; Qiu, Y.; Kamon, T.; Arai, M.; Tun, H.W.; Yoshimitsu, T. An integrated approach to the discovery of potent agelastatin A analogues for brain tumors: Chemical synthesis and biological, physicochemical and CNS pharmacokinetic analyses. MedChemComm 2013, 4, 1093–1098. [Google Scholar] [CrossRef]
- Antropow, A.H.; Xu, K.; Buchsbaum, R.J.; Movassaghi, M. Synthesis and Evaluation of Agelastatin Derivatives as Potent Modulators for Cancer Invasion and Metastasis. J. Org. Chem. 2017, 82, 7720–7731. [Google Scholar] [CrossRef]
- Stout, E.P.; Choi, M.Y.; Castro, J.E.; Molinski, T.F. Potent Fluorinated Agelastatin Analogues for Chronic Lymphocytic Leukemia: Design, Synthesis, and Pharmacokinetic Studies. J. Med. Chem. 2014, 57, 5085–5093. [Google Scholar] [CrossRef]
- McClary, B.; Zinshteyn, B.; Meyer, M.; Jouanneau, M.; Pellegrino, S.; Yusupova, G.; Schuller, A.; Reyes, J.C.P.; Lu, J.; Guo, Z.; et al. Inhibition of eukaryotic translation by the antitumor natural product Agelastatin A. Cell Chem. Biol. 2017, 24, 605–613. [Google Scholar] [CrossRef]
- Pietra, F. Fighting cancer with translation inhibitors: A quantum mechanics view on the complex between the antitumor marine alkaloid agelastatin A and the yeast 80S ribosome. Struct. Chem. 2021, 32, 1407–1414. [Google Scholar] [CrossRef]
- Tilvi, S.; Moriou, C.; Martin, M.T.; Gallard, J.F.; Sorres, J.; Patel, K.; Petek, S.; Debitus, C.; Ermolenko, L.; Al-Mourabit, A. Agelastatin E, agelastatin F, and benzosceptrin C from the marine sponge Agelas dendromorpha. J. Nat. Prod. 2010, 73, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.W.; Jímenez, D.R.; Molinski, T.F. Agelastatins C and D, new pentacyclic bromopyrroles from the sponge Cymbastela sp. and potent arthropod toxicity of (−)-agelastatin A. J. Nat. Prod. 1998, 61, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yakushijin, K.; Horne, D.A. Synthesis of C11N5 Marine Sponge Alkaloids: (±)-Hymenin, Stevensine, Hymenialdisine, and Debromohymenialdisine. J. Org. Chem. 1997, 62, 456–464. [Google Scholar] [CrossRef] [PubMed]
- March, J. Advanced Organic Chemistry, 4th ed.; Wiley-Interscience Publication: New York, NY, USA, 1992; pp. 531–534, 566–567. ISBN 10 0471581488. [Google Scholar]
- Wu, P.; Nielsen, T.E. Scaffold Diversity from N-Acyliminium Ions. Chem. Rev. 2017, 117, 7811–7856. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M. Recent advances in the synthesis of saturated nitrogen heterocycles using n-acyliminium ion intermediates. In Targets in Heterocyclic Systems, 1st ed.; Attanasi, O.A., Spinelli, D., Eds.; Società Chimica Italiana: Roma, Italy, 2002; Volume 6, pp. 99–135. ISBN 88-86208-23-5. [Google Scholar]
- Langley, D.R. Mechanism of action and molecular modeling for esperamicin, calicheamicin γ1I and dynemicin A. In Enediyne Antibiotics as Antitumor Agents; Borders, D.B., Doyle, T.W., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 239–282. ISBN 1849730504. [Google Scholar]
- D’Ambrosio, M.; Guerriero, A.; Pietra, F. Isolation from the Mediterranean stoloniferan coral Sarcodictyon roseum of sarcodictyin C, D, E, and F, novel diterpenoidic alcohols esterified by (E)- or (Z)-N(1)-methylurocanic acid. Failure of the carbon- skeleton type as a classification criterion. Helv. Chim. Acta 1988, 71, 964–976. [Google Scholar] [CrossRef]
- Neukirch, H.; Guerriero, A.; D’Ambrosio, M. Transannular Cyclization in Cyclodecenes: The Case Study of Melampolides. Eur. J. Org. Chem. 2003, 2003, 3969–3975. [Google Scholar] [CrossRef]
- Freund, R.R.A.; Gobrecht, P.; Fischer, D.; Arndt, H.-D. Advances in chemistry and bioactivity of parthenolide. Nat. Prod. Rep. 2020, 37, 541–565. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Chen, J.S.; Dalby, S.M. From nature to the laboratory and into the clinic. Bioorg. Med. Chem. 2009, 17, 2290–2303. [Google Scholar] [CrossRef]
- D’Ambrosio, M. The N-Alkylation of Agelastatin A Modulates Its Chemical Reactivity. In Proceedings of the 22nd Tetrahedron Symposium: Catalysis for a Sustainable World, Lisbon, Portugal, 28 June–1 July 2022. [Google Scholar]



| Reaction | Conditions | Compounds by 1H-NMR | Compound, tR and Possible [M + H]+ (m/z) by LC-DAD-MS Experiments. Style by UV Bands. | |||
|---|---|---|---|---|---|---|
| - | Mix of known compounds | 6e 1 1d 1m 1e 2m 2e | 6e/14.5 1/15.9 1d/17.6 1m/19.1 1e/21.3 2m/25.5 2e/29.2 | |||
| R6 | 2e + 0.1eq.MsOH, CHCl3, 70°, 24 h | 4e (2%) 3e (90%) 2e (10%) | 2’e/19.9 | 4e/24.2 | 3e/25.9 | 2e/28.6 |
| R7 | 2e + 0.5eq.MsOH, CHCl3, 70°, 24 h | 3e~1e 40% 4e 10% 6e 8% 5e (2%) | 1’e/13.6 (369–371) 1e/20.3 (369–371) | 6e/14.1 (273) 4e/23.9 (351–353) | 5e/14.4 (273) 3e/25.7 (351–353) | 2’e/19.5 (383–385) 2e/28.4 (383–385) |
| R8 | 2e + 1.1eq.MsOD, CDCl3, 70°, 1.5 h | 6e (70%), 5e (30%) | 6e/14.1 (273) | 5e/14.3 (273) | ||
| R9 | 2e + 0.8eq.MsOH, CHCl3, 70°, 1.2 h | (First aliquot after 15’) | 6e/14.1 4e/23.9 | 5e/14.3 3e/25.7 | 2e/28.3 | |
| 6e (95%), 5e (5%) | 6e/14.1 (273) | 5e/14.3 (273) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosio, M. The N-Alkylation of Agelastatin A Modulates Its Chemical Reactivity. Molecules 2023, 28, 6821. https://doi.org/10.3390/molecules28196821
D’Ambrosio M. The N-Alkylation of Agelastatin A Modulates Its Chemical Reactivity. Molecules. 2023; 28(19):6821. https://doi.org/10.3390/molecules28196821
Chicago/Turabian StyleD’Ambrosio, Michele. 2023. "The N-Alkylation of Agelastatin A Modulates Its Chemical Reactivity" Molecules 28, no. 19: 6821. https://doi.org/10.3390/molecules28196821