Antioxidant and Anti-Inflammatory Activities of Phenolic Acid-Rich Extract from Hairy Roots of Dracocephalum moldavica
Abstract
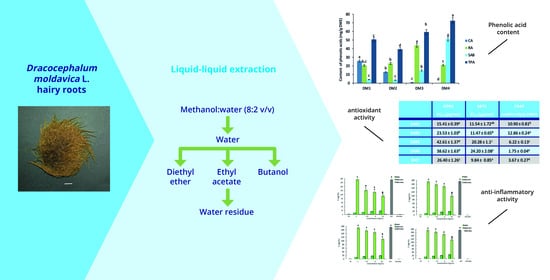
:1. Introduction
2. Results
2.1. Determination of Phenolic Acids Content
2.2. Biological Activity of D. moldavica Hairy Roots
2.2.1. Antioxidant Activity
2.2.2. Cytotoxicity and Anti-Inflammatory Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of Extracts
4.2. Quantitative HPLC Analysis
4.3. Antioxidant Assays
4.4. Cell Culture
4.5. Cell Stimulation Condition
4.6. MTT Reduction Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, Y.P.; Turdimatovich, T.O.; Nuraliev, M.S.; Lazarević, P.; Drew, B.T.; Xiang, C.L. Phylogeny and biogeography of the northern temperate genus Dracocephalum s.l. (Lamiaceae). Cladistics 2022, 38, 429–451. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Sikora, V.; Brdar-Jokanović, M.; Kiprovski, B.; Popović, V.; Koren, A.; Puvača, N. Dracocephalum moldavica: Cultivation, chemical composition and biological activity. J. Agron. Technol. Eng. Manag. 2019, 2, 153–167. [Google Scholar]
- Yu, H.; Liu, M.; Liu, Y.; Qin, L.; Jin, M.; Wang, Z. Antimicrobial activity and mechanism of action of Dracocephalum moldavica L. extracts against clinical isolates of Staphylococcus aureus. Front. Microbiol. 2019, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Amita, P.; Sahalini, T. A review on pharmacognosy, pre-phytochemistry and pharmacological analysis of Tridax procumbens. PharmaTutor 2014, 2, 78–86. [Google Scholar]
- Weremczuk-Jeżyna, I.; Grzegorczyk-Karolak, I.; Frydrych, B.; Królicka, A.; Wysokińska, H. Hairy roots of Dracocephalum moldavica: Rosmarinic acid content and antioxidant potential. Acta Physiol. Plant. 2013, 35, 2095–2103. [Google Scholar] [CrossRef]
- Ramana, K.V.; Singhal, S.S.; Reddy, A.B. Therapeutic potential of natural pharmacological agents in treatment of human diseases. BioMed Res. Int. 2014, 2014, 573452. [Google Scholar] [CrossRef]
- Kapadia, P.; Newell, A.S.; Cunningham, J.; Roberts, M.R.; Hardy, J.G. Extraction of high-value chemicals from plants for technical and medical applications. Int. J. Mol. Sci. 2022, 23, 10334. [Google Scholar] [CrossRef]
- Weremczuk-Jezyna, I.; Skała, E.; Olszewska, M.A.; Kiss, A.K.; Balcerczak, E.; Wysokińska, H.; Kicel, A. The identification and quantitative determination of rosmarinic acid and salvianolic acid B in hairy root cultures of Dracocephalum forrestii W.W. Smith. Ind. Crops Prod. 2016, 91, 125–131. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Kochan, E.; Szymczyk, P.; Lisiecki, P.; Kuźma, Ł.; Grzegorczyk-Karolak, I. The antioxidant and antimicrobial properties of phenol-rich extracts of Dracocephalum forrestii W.W. Smith shoot cultures grown in the nutrient sprinkle bioreactor. Phytochem. Lett. 2019, 30, 254–260. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Olszewska, M.A.; Owczarek, A. Phytochemical profile and antioxidant activity of aerial and underground parts of Salvia bulleyana Diels. plants. Metabolites 2020, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Zeroual, A.; Eloutassi, N.; Chaouch, M.; Chaqroune, A. Antimicrobial, antioxidant activity, and chemical composition of Origanum compactum Benth from Taounate Province, North Morocco. Asian J. Pharm. Clin. Res. 2020, 3, 126–131. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of polyphenols from roamatic and medicinal plants: An overview of the methods and the effect of extraction parameters. In Polyphenols in Plants, 2nd ed.; Ronald, W., Ed.; Academic Press: London, UK, 2019; pp. 243–260. [Google Scholar] [CrossRef]
- Queimada, A.J.; Mota, F.L.; Pinho, S.P.; Macedo, E.A. Solubilities of biologically active phenolic compounds: Measurements and modelling. J. Phys. Chem. B 2009, 113, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Xu, H.; Cui, Y.; Song, D.; Feng, Y.Q. Improved liquid-liquid-liquid microextraction method and its application to analysis of four phenolic compounds in water samples. J. Chromatogr. A 2008, 1203, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Du, M.; Ding, J.; Liu, P. Salvianolic acid B attenuates the inflammatory response in atherosclerosis by regulating MAPKs/NF-κB signaling pathways in LDLR−/− mice and RAW264.7 cells. Int. J. Immunopathol. Pharmacol. 2022, 36, 03946320221079468. [Google Scholar] [CrossRef]
- Napoli, E.; Ruberto, G.; Carrubba, A.; Sarno, M.; Muscarà, C.; Speciale, A.; Cristani, M.; Cimino, F.; Saija, A. Phenolic profiles, antioxidant and anti-inflammatory activities of hydrodistillation wastewaters from five Lamiaceae species. Molecules 2022, 27, 7427. [Google Scholar] [CrossRef]
- Moradi, H.; Ghavam, M.; Tavili, A. Study of antioxidant activity and some herbal compounds of Dracocephalum kotschyi Boiss. in different ages of growth. Biotechnol. Rep. 2020, 25, e00408. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Okhlopkova, Z.M.; Zulfugarov, I.S. Chemical composition and antioxidant activity of Tánara Ótó (Dracocephalum palmatum Stephan), a medicinal plant used by the North-Yakutian nomads. Molecules 2013, 18, 14105–14121. [Google Scholar] [CrossRef] [PubMed]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M.; Chabani, Z.; Farag, M.A.; Domínguez, R. Measurement of antioxidant capacity of meat and meat products: Methods and applications. Molecules 2021, 26, 3880. [Google Scholar] [CrossRef]
- Khodaei, M.; Amanzadeh, Y.; Faramarzi, M.A.; Hamedani, M.P.; Adhami, H.R. Cholinesterase inhibitory, anti-oxidant and anti-tyrosinase activities of three Iranian species of Dracocephalum. J. Pharmacogn. 2019, 6, 25–31. [Google Scholar] [CrossRef]
- Xavier, J.R.; Chaurasia, O.P.; Vajpayee, P.K.; Bajpai, P.K.; Muthaiah, P.M. Antioxidative activity and phytochemical investigation on a high altitude medicinal plant Dracocephalum heterophyllum Benth. Phcog. J. 2009, 1, 246–251. [Google Scholar]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical profile and antioxidant activity of Lavandula angustifolia and Lavandula x intermedia cultivars extracted with different methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef]
- Alu’datt, M.; Rababah, T.; Alhamad, M.N.; Ereifej, K.; Al-Mahasenh, M.; Brewer, S.; Rawshadeh, M. Optimization extraction conditions for phenolic compounds, antioxidant and inhibitory activities of angiotensin I-converting enzyme (ACE), α-glucoside and α-amylose from Mentha spicata L. J. Food Biochem. 2016, 40, 335–364. [Google Scholar] [CrossRef]
- Pouolios, E.; Giagins, C.; Vasios, G.K. Current state of art on the antioxidant activity of sage (Salvia spp.) and its bioactive components. Planta Med. 2020, 86, 224–238. [Google Scholar] [CrossRef]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure- activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Moazzen, A.; Oztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 2022, 8, e10467. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wu, C.H.; Luo, M.H.; Huang, Y.J.; Wang, C.N.; Shiao, M.S.; Huang, Y.T. In vitro protective effects of salvianolic acid B on primary hepatocytes and hepatic stellate cells. J. Ethnopharmacol. 2006, 105, 215–222. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Štefan, M.B.; Alegro, A.; Kőszegi, T.; Petrik, J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, S.; Li, H.; Jiang, X.; Yin, P.; Wan, C.; Liu, X.; Liu, F.; Xu, J. The protective effect of caffeic acid against inflammation injury of primary bovine mammary epithelial cells induced by lipopolysaccharide. J. Dairy Sci. 2014, 97, 2856–2865. [Google Scholar] [CrossRef] [PubMed]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Cappello, M.S.; Dugay, A.; Deguin, B.; Lauria, G.; Cappello, A.R.; Tundis, R. New insights into the antioxidant and anti-inflammatory effects of Italian Salvia officinalis leaf and flower extracts in lipopolysaccharide and tumor-mediated inflammation models. Antioxidants 2021, 10, 311. [Google Scholar] [CrossRef]
- Tantipaiboonwong, P.; Pintha, K.; Chaiwangyen, W.; Suttajit, M.; Khanaree, C.; Khantamat, O. Bioefficacy of Nga-Mon (Perilla frutescens) fresh and dry leaf: Assessment of antioxidant, antimutagenicity, and anti-inflammatory potential. Plants 2023, 12, 2210. [Google Scholar] [CrossRef]
- Gonciarz, W.; Piątczak, E.; Chmiela, M. The influence of Salvia cadmica Boiss. extracts on the M1/M2 polarization of macrophages primed with Helicobacter pylori lipopolysaccharide in conjunction with NF-kappa B activation, production of cytokines, phagocytic activity and total DNA methylation. J. Ethnopharmacol. 2023, 310, 116386. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Shan, W.; Gao, L.; Gao, D.; Hu, Y.; Wang, G.; Zhang, N.; Li, Z.; Tian, X.; Xu, X.; et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 16013. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, W.; Jin, L. Caffeic acid alleviates inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes by inhibiting phosphorylation of IκB kinase α/β and IκBα. Int. Immunopharmacol. 2017, 48, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hu, C.; Wu, L.; Xu, L.; Jiang, W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-kappaB signalling in H22 tumor-bearing mice. J. Pharmacol. Sci. 2016, 132, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Taïlé, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.P. Medicinal plant polyphenols attenuate oxidative stress and improve inflammatory and vasoactive markers in cerebral endothelial cells during hyperglycemic condition. Antioxidants 2020, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mu, S.; Xia, H.; Lou, H.; Zhu, F.; Sun, L. Anti-inflammatory activities and potential mechanisms of phenolic acids isolated from Salvia miltiorrhiza f. alba roots in THP-1 macrophages. J. Ethnopharmacol. 2018, 222, 201–207. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.L.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Baeuerle, P.A. Ikappa B-NF-kappa B structures: At the interface of inflammation control. Cell 1998, 95, 729–731. [Google Scholar] [CrossRef]
- Kabe, Y.; Ando, K.; Hirao, S.; Yoshida, M.; Handa, H. Redox regulation of NF-kappaB activation: Distinct redox regulation between the cytoplasm and the nucleus. Antioxid. Redox Signal. 2005, 7, 395. [Google Scholar] [CrossRef]
- Oers, N.S.C.V.; Chen, Z.J. Kinasing and upping down the NF-kappa B trail. Science 2005, 308, 65–66. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kiss, A.K. Determination of the phenolic profile and antioxidant properties of Salvia viridis L. shoots: A comparison of aqueous and hydroethanolic extracts. Molecules 2018, 23, 1468. [Google Scholar] [CrossRef]
- Gonciarz, W.; Matusiak, A.; Rudnicka, K.; Rechciński, T.; Chałubiński, M.; Czkwianianc, E.; Broncel, M.; Gajewski, A.; Chmiela, M. Autoantibodies to a specific peptide epitope of human Hsp60 (ATVLA) with homology to Helicobacter pylori HspB in H. pylori-infected patients. Apmis 2019, 127, 139–149. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for Cytotoxicity: In Vitro Methods. International Organization for Standardization: Geneve, Switzerland, 2009.
- Weremczuk-Jeżyna, I.; Lisiecki, P.; Gonciarz, W.; Kuźma, Ł.; Szemraj, M.; Chmiela, M.; Grzegorczyk-Karolak, I. Transformed shoots of Dracocephalum forrestii W.W. Smith from different bioreactor systems as a rich source of natural phenolic compounds. Molecules 2020, 25, 4533. [Google Scholar] [CrossRef] [PubMed]




| DPPH EC50 (µg/mL) | ABTS EC50 (µg/mL) | FRAP (mM Fe (II)/g DWE) | |
|---|---|---|---|
| DM1 | 15.41 ± 0.39 b | 11.54 ± 1.72 bc | 10.90 ± 0.81 b |
| DM2 | 23.53 ± 1.03 c | 11.47 ± 0.65 c | 12.86 ± 0.24 a |
| DM3 | 42.61 ± 1.37 e | 20.28 ± 1.1 d | 6.22 ± 0.13 c |
| DM4 | 38.62 ± 1.63 e | 24.20 ± 2.08 d | 1.75 ± 0.04 f |
| BHT | 26.40 ± 1.26 d | 9.84 ± 0.85 b | 3.67 ± 0.27 e |
| Trolox | 4.72 ± 0.03 a | 4.44 ± 0.03 a | 5.23 ± 0.10 d |
| CA | RA | SAB | TPAC | |
|---|---|---|---|---|
| DPPH | −0.971 | 0.651 | 0.627 | 0.689 |
| ABTS | −0.875 | 0.321 | 0.889 | 0.931 |
| FRAP | 0.762 | −0.182 | −0.930 | −0.984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weremczuk-Jeżyna, I.; Gonciarz, W.; Grzegorczyk-Karolak, I. Antioxidant and Anti-Inflammatory Activities of Phenolic Acid-Rich Extract from Hairy Roots of Dracocephalum moldavica. Molecules 2023, 28, 6759. https://doi.org/10.3390/molecules28196759
Weremczuk-Jeżyna I, Gonciarz W, Grzegorczyk-Karolak I. Antioxidant and Anti-Inflammatory Activities of Phenolic Acid-Rich Extract from Hairy Roots of Dracocephalum moldavica. Molecules. 2023; 28(19):6759. https://doi.org/10.3390/molecules28196759
Chicago/Turabian StyleWeremczuk-Jeżyna, Izabela, Weronika Gonciarz, and Izabela Grzegorczyk-Karolak. 2023. "Antioxidant and Anti-Inflammatory Activities of Phenolic Acid-Rich Extract from Hairy Roots of Dracocephalum moldavica" Molecules 28, no. 19: 6759. https://doi.org/10.3390/molecules28196759