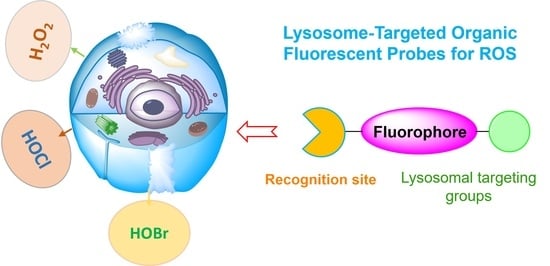
Recent Development of Lysosome-Targeted Organic Fluorescent Probes for Reactive Oxygen Species
Abstract
:1. Introduction
1.1. Fluorescence Imaging and Fluorescent Probes
1.2. Lysosomes and Lysosome-Targeted Fluorescent Probes
2. Lysosome-Targeted Fluorescent Probes for ROS
2.1. Hydrogen Peroxide (H2O2)
2.2. Hypochlorous Acid (HOCl)
2.3. Hypobromous Acid (HOBr)
3. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Ren, W.X.; Hou, J.T.; Won, M.; An, J.; Chen, X.Y.; Shu, J.; Kim, J.S. Fluorescence imaging of pathophysiological microenvironments. Chem. Soc. Rev. 2021, 50, 8887–8902. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Chen, G.C.; Zhang, Y.J.; Wu, F.; Wang, Q.B. Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications. J. Am. Chem. Soc. 2020, 142, 14789–14804. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Gao, F.C.; Wang, Y.D.; Li, H.; Zhang, J.; Sun, Z.W.; Jiang, Y.Y. Fluorescent Organic Small Molecule Probes for Bioimaging and Detection Applications. Molecules 2022, 27, 8421. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Ha, J.; Cho, M.; Li, H.D.; Swamy, K.M.K.; Yoon, J. Recent developments of BODIPY-based colorimetric and fluorescent probes for the detection of reactive oxygen/nitrogen species and cancer diagnosis. Coord. Chem. Rev. 2021, 439, 213936. [Google Scholar] [CrossRef]
- Li, W.; Schierle, G.S.K.; Lei, B.F.; Liu, Y.L.; Kaminski, C.F. Fluorescent Nanoparticles for Super-Resolution Imaging. Chem. Rev. 2022, 122, 12495–12543. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Deng, B.; Wang, J.Y.; Kong, X.; Liu, Z.R.; Zhou, K.; He, L.; Lin, W. A fast responsive two-photon fluorescent probe for imaging H2O2 in lysosomes with a large turn-on fluorescence signal. Biosens. Bioelectron. 2016, 79, 237–243. [Google Scholar] [CrossRef]
- Qi, S.J.; Kim, S.; Nguyen, V.N.; Kim, Y.; Niu, G.L.; Kim, G.M.; Kim, S.J.; Park, S.; Yoon, J. Highly Efficient Aggregation-Induced Red-Emissive Organic Thermally Activated Delayed Fluorescence Materials with Prolonged Fluorescence Lifetime for Time-Resolved Luminescence Bioimaging. ACS Appl. Mater. Interfaces 2020, 12, 51293–51301. [Google Scholar] [CrossRef]
- Liu, L.Y.; Zhao, Y.; Zhang, N.; Wang, K.N.; Tian, M.; Pan, Q.; Lin, W. Ratiometric Fluorescence Imaging for the Distribution of Nucleic Acid Content in Living Cells and Human Tissue Sections. Anal. Chem. 2021, 93, 1612–1619. [Google Scholar] [CrossRef]
- Hickey, S.M.; Ung, B.; Bader, C.; Brooks, R.; Lazniewska, J.; Johnson, I.R.D.; Sorvina, A.; Logan, J.; Martini, C.; Moore, C.R.; et al. Fluorescence Microscopy-An Outline of Hardware, Biological Handling, and Fluorophore Considerations. Cells 2021, 11, 35. [Google Scholar] [CrossRef]
- Lelek, M.; Gyparaki, M.T.; Beliu, G.; Schueder, F.; Griffie, J.; Manley, S.; Jungmann, R.; Sauer, M.; Lakadamyali, M.; Zimmer, C. Single-molecule localization microscopy. Nat. Rev. Dis. Primers 2021, 1, 39. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications. Molecules 2022, 27, 8628. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhu, T.; Xu, J.Y.; Zhao, Y.; Kuang, Y.W.; Sun, M.N.; Chen, Y.Q.; He, W.; Wang, Z.X.; Jiang, T.W.; et al. Organic Fluorescent Probes for Monitoring Micro-Environments in Living Cells and Tissues. Molecules 2023, 28, 3455. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sedgwick, A.C.; Hirao, T.; Sessler, J.L. Supramolecular Fluorescent Sensors: An Historical Overview and Update. Coord. Chem. Rev. 2021, 427, 213560. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, D.H.; Hong, J.I.; Yoon, J. Chemosensors for pyrophosphate. Acc. Chem. Res. 2009, 42, 23–31. [Google Scholar] [CrossRef]
- Munan, S.; Ali, M.; Yadav, R.; Mapa, K.; Samanta, A. PET- and ICT-Based Ratiometric Probe: An Unusual Phenomenon of Morpholine-Conjugated Fluorophore for Mitochondrial pH Mapping during Mitophagy. Anal. Chem. 2022, 94, 11633–11642. [Google Scholar] [CrossRef]
- Jing, X.Y.; Yu, F.Q.; Lin, W.Y. A PET-based lysosome-targeted turn-on fluorescent probe for the detection of H2S and its bioimaging application in living cells and zebrafish. New J. Chem. 2019, 43, 16796–16800. [Google Scholar] [CrossRef]
- Du, C.C.; Fu, S.B.; Wang, X.H.; Sedgwick, A.C.; Zhen, W.; Li, M.J.; Li, X.Q.; Zhou, J.; Wang, Z.; Wang, H.Y.; et al. Diketopyrrolopyrrole-based fluorescence probes for the imaging of lysosomal Zn2+ and identification of prostate cancer in human tissue. Chem. Sci. 2019, 10, 5699–5704. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Yoon, J. Recent Advances in Development of Chiral Fluorescent and Colorimetric Sensors. Chem. Rev. 2014, 114, 4918–4959. [Google Scholar] [CrossRef]
- Li, Z.Y.; Cui, X.L.; Xiao, M.M.; Miao, J.Y.; Zhao, B.X.; Lin, Z.M. An FRET-ICT-based ratiometric fluorescent and colorimetric probe for pH monitoring in lysosomes and water. Dyes Pigm. 2021, 193, 109481. [Google Scholar] [CrossRef]
- Abeywickrama, C.S. Large Stokes shift benzothiazolium cyanine dyes with improved intramolecular charge transfer (ICT) for cell imaging applications. Chem. Commun. 2022, 58, 9855–9869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huo, F.J.; Zhang, W.J.; Chao, J.B.; Yin, C.X. Ultra-pH-sensitive sensor for visualization of lysosomal autophagy, drug-induced pH alteration and malignant tumors microenvironment. Sens. Actuators B Chem. 2021, 345, 130393. [Google Scholar] [CrossRef]
- Roy, B.; Mengji, R.; Roy, S.; Pal, B.; Jana, A.; Singh, N.D.P. NIR-Responsive Lysosomotropic Phototrigger: An “AIE plus ESIPT” Active Naphthalene-Based Single-Component Photoresponsive Nanocarrier with Two-Photon Uncaging and Real-Time Monitoring Ability. ACS Appl. Mater. Interfaces 2022, 14, 4862–4870. [Google Scholar] [CrossRef] [PubMed]
- Abeywickrama, C.S.; Bertman, K.A.; Mcdonald, L.J.; Alexander, N.; Dahal, D.; Baumann, H.J.; Salmon, C.R.; Wesdemiotis, C.; Konopka, M.; Tessier, C.A.; et al. Synthesis of highly selective lysosomal markers by coupling 2-(2′-hydroxyphenyl)benzothiazole (HBT) with benzothiazolium cyanine (Cy): The impact of substituents on selectivity and optical properties. J. Mater. Chem. B 2019, 7, 7502–7514. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Nair, A.V.; Shah, S.S.; Ray, S.; Singh, N.D.P. ESIPT-, AIE-, and AIE plus ESIPT-Based Light-Activated Drug Delivery Systems and Bioactive Donors for Targeted Disease Treatment. J. Med. Chem. 2023, 66, 3732–3745. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.J.; Wu, Q.; Yin, C.X.; Zhang, W.J.; Zhang, Y.B. A high efficient and lysosome targeted “off-on” probe for sulfite based on nucleophilic addition and ESIPT. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 429–435. [Google Scholar] [CrossRef]
- Shi, X.; Yan, N.; Niu, G.; Sung, S.H.P.; Liu, Z.; Liu, J.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, W.X.; Sung, H.H.; et al. In vivo monitoring of tissue regeneration using a ratiometric lysosomal AIE probe. Chem. Sci. 2020, 11, 3152–3163. [Google Scholar] [CrossRef]
- Hong, Y.X.; Wang, H.; Xue, M.J.; Zhang, P.S.; Liu, W.Q.; Chen, S.; Zeng, R.J.; Cui, J.X.; Gao, Y.; Chen, J. Rational design of ratiometric and lysosome-targetable AIE dots for imaging endogenous HClO in live cells. Mater. Chem. Front. 2019, 3, 203–208. [Google Scholar] [CrossRef]
- Shi, W.J.; Chen, R.; Yang, J.R.; Wei, Y.F.; Guo, Y.H.; Wang, Z.Z.; Yan, J.W.; Niu, L. Novel Meso-Benzothiazole-Substituted BODIPY-Based AIE Fluorescent Rotor for Imaging Lysosomal Viscosity and Monitoring Autophagy. Anal. Chem. 2022, 94, 14707–14715. [Google Scholar] [CrossRef]
- Zhu, K.; Lv, T.; Qin, T.; Huang, Y.; Wang, L.; Liu, B. A flavonoid-based fluorescent probe enables the accurate quantification of human serum albumin by minimizing the interference from blood lipids. Chem. Commun. 2019, 55, 13983–13986. [Google Scholar] [CrossRef]
- Bertman, K.A.; Abeywickrama, C.S.; Baumann, H.J.; Alexander, N.; McDonald, L.; Shriver, L.P.; Konopka, M.; Pang, Y. A fluorescent flavonoid for lysosome detection in live cells under “wash free” conditions. J. Mater. Chem. B 2018, 6, 5050–5058. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kim, H.; Xu, F.; Han, J.; Yao, Q.; Wang, J.; Pu, K.; Peng, X.; Yoon, J. Activity-based NIR fluorescent probes based on the versatile hemicyanine scaffold: Design strategy, biomedical applications, and outlook. Chem. Soc. Rev. 2022, 51, 1795–1835. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Wang, M.; Liu, W.; Liu, L.; Xu, P. Activity-Based Fluorescent Probes Based on Hemicyanine for Biomedical Sensing. Molecules 2022, 27, 7750. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, L.; Xu, Q.; Chen, X.; Yoon, J. Design Principles, Sensing Mechanisms, and Applications of Highly Specific Fluorescent Probes for HOCl/OCl−. Acc. Chem. Res. 2019, 52, 2158–2168. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Heo, S.; Kim, S.; Swamy, K.M.K.; Ha, J.; Park, S.; Yoon, J. A thiocoumarin-based turn-on fluorescent probe for hypochlorite detection and its application to live-cell imaging. Sens. Actuators B Chem. 2020, 317, 128213. [Google Scholar] [CrossRef]
- Cho, M.; Nguyen, V.N.; Yoon, J. Simultaneous Detection of Hypochlorite and Singlet Oxygen by a Thiocoumarin-Based Ratiometric Fluorescent Probe. ACS Meas. Sci. Au. 2022, 2, 219–223. [Google Scholar] [CrossRef]
- Xu, H.X.; Ren, D.J. Lysosomal Physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Baek, G.; Qi, S.; Heo, S.; Yim, Y.; Yoon, J. A lysosome-localized thionaphthalimide as a potential heavy-atom-free photosensitizer for selective photodynamic therapy. Dyes Pigm. 2020, 177, 108265. [Google Scholar] [CrossRef]
- Choi, N.E.; Lee, J.Y.; Park, E.C.; Lee, J.H.; Lee, J. Recent Advances in Organelle-Targeted Fluorescent Probes. Molecules 2021, 26, 217. [Google Scholar] [CrossRef]
- Hou, J.T.; Yu, K.K.; Sunwoo, K.; Kim, W.Y.; Koo, S.; Wang, J.Y.; Ren, W.X.; Wang, S.; Yu, X.Q.; Kim, J.S. Fluorescent Imaging of Reactive Oxygen and Nitrogen Species Associated with Pathophysiological Processes. Chem 2020, 6, 832–866. [Google Scholar] [CrossRef]
- Gao, P.; Pan, W.; Li, N.; Tang, B. Fluorescent probes for organelle-targeted bioactive species imaging. Chem. Sci. 2019, 10, 6035–6071. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Sedgwick, A.C.; Sun, X.L.; Bull, S.D.; He, X.P.; James, T.D. Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.J.; Wang, Z.; Zhou, J.Y.; Zhu, M.G.; Liu, J.; James, T.D. Recent progress in the development of fluorescent probes for imaging pathological oxidative stress. Chem. Soc. Rev. 2023, 52, 3873–3926. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.C.; Aleyasin, H.; Dickinson, B.C.; Haskew-Layton, R.E.; Ratan, R.R. Recent advances in hydrogen peroxide imaging for biological applications. Cell Biosci. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Hu, J.J.; Zhao, Q.A.; Yang, D. Fluorescent probes for in vitro and in vivo quantification of hydrogen peroxide. Chem. Sci. 2020, 11, 11989–11997. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Jiao, Y.; Ma, C.M.; Duan, C.Y. A Novel Fluorescent Probe for Hydrogen Peroxide and Its Application in Bio-Imaging. Molecules 2021, 26, 3352. [Google Scholar] [CrossRef]
- Li, S.R.; Xiao, Y.S.; Chen, C.; Jia, L.X. Recent Progress in Organic Small-Molecule Fluorescent Probe Detection of Hydrogen Peroxide. Acs Omega 2022, 7, 15267–15274. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, T.; Yang, L.; Yuan, L.; Liang, L.; Xu, P. Revelation of the dynamic progression of hypoxia-reoxygenation injury by visualization of the lysosomal hydrogen peroxide. Biochem. Biophys. Res. Commun. 2017, 486, 904–908. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, S.Q.; Ren, J.; Wu, C.L.; Zhao, Y.B. A lysosome-locating and acidic pH-activatable fluorescent probe for visualizing endogenous H2O2 in lysosomes. Analyst 2017, 142, 4522–4528. [Google Scholar] [CrossRef]
- Reja, S.I.; Gupta, M.; Gupta, N.; Bhalla, V.; Ohri, P.; Kaur, G.; Kumar, M. A lysosome targetable fluorescent probe for endogenous imaging of hydrogen peroxide in living cells. Chem. Commun. 2017, 53, 3701–3704. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, W.J.; Wei, X.R.; Xu, Y.J.; Sun, R.; Ge, J.F. Near-infrared and lysosome-targetable fluorescent probe based on phenoxazinium for hydrogen peroxide detection. Anal. Methods 2018, 10, 3754–3758. [Google Scholar] [CrossRef]
- Kim, D.; Kim, G.; Nam, S.J.; Yin, J.; Yoon, J. Visualization of Endogenous and Exogenous Hydrogen Peroxide Using A Lysosome-Targetable Fluorescent Probe. Sci. Rep. 2015, 5, 8488. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.R.; Peng, Q.Y.; Wan, D.; Yu, C.; Zhang, Y.; Hou, Y.; Luo, Q.; Li, X.; Zhang, S.H.; Xie, L.; et al. Construction of a lysosome-targetable ratiometric fluorescent probe for H2O2 tracing and imaging in living cells and an inflamed model. RSC Adv. 2021, 11, 24032–24037. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.N.; Xu, J.H.; Ma, Q.J.; Mao, G.J.; Zhang, J.; Li, L.K.; Liu, S.Z. A new lysosome-targeted fluorescent probe for hydrogen peroxide based on a benzothiazole derivative. Methods 2023, 215, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Xiao, S.Y.; Gong, X.L.; Zhu, X.; Wang, Y.W.; Peng, Y. A Near-Infrared Fluorescent Probe for Recognition of Hypochlorite Anions Based on Dicyanoisophorone Skeleton. Molecules 2023, 28, 402. [Google Scholar] [CrossRef]
- Hou, J.T.; Kwon, N.; Wang, S.; Wang, B.Y.; He, X.J.; Yoon, J.; Shen, J.L. Sulfur-based fluorescent probes for HOCl: Mechanisms, design, and applications. Coord. Chem. Rev. 2022, 450, 214232. [Google Scholar] [CrossRef]
- Zhang, B.B.; Yang, X.P.; Zhang, R.; Liu, Y.; Ren, X.L.; Xian, M.; Ye, Y.; Zhao, Y.F. Lysosomal-Targeted Two-Photon Fluorescent Probe to Sense Hypochlorous Acid in Live Cells. Anal. Chem. 2017, 89, 10384–10390. [Google Scholar] [CrossRef]
- Jiao, X.J.; Liu, C.; Wang, Q.; Huang, K.; He, S.; Zhao, L.C.; Zeng, X.S. Fluorescence probe for hypochlorous acid in water and its applications for highly lysosome-targetable live cell imaging. Anal. Chim. Acta 2017, 969, 49–56. [Google Scholar] [CrossRef]
- Ma, H.; Song, B.; Wang, Y.X.; Liu, C.L.; Wang, X.; Yuan, J.L. Development of organelle-targetable europium complex probes for time-gated luminescence imaging of hypochlorous acid in live cells and animals. Dyes Pigm. 2017, 140, 407–416. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wu, L.; Liu, C.Y.; Guo, B.P.; Zhu, B.C.; Wang, Z.K.; Duan, Q.X.; Ma, Z.M.; Zhang, X.L. A highly specific and ultrasensitive fluorescent probe for basal lysosomal HOCl detection based on chlorination induced by chlorinium ions (Cl+). J. Mater. Chem. B 2017, 5, 3377–3382. [Google Scholar] [CrossRef]
- Ren, M.G.; Nie, J.; Deng, B.B.; Zhou, K.; Wang, J.Y.; Lin, W.Y. A fluorescent probe for ratiometric imaging of exogenous and intracellular formed hypochlorous acid in lysosomes. New J. Chem. 2017, 41, 5259–5262. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, J.L.; Cheng, G.H.; Ghazali, S.; Du, J.J.; Peng, X.J. Fluorescence completely separated ratiometric probe for HClO in lysosomes. Sens. Actuators B Chem. 2017, 246, 293–299. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, Z.M.; Zhang, Y.R.; Su, L.; Miao, J.Y.; Zhao, B.X. A lysosome-targeted ratiometric fluorescent probe for detection of hypochlorous acid in living cells. Sens. Actuators B Chem. 2017, 247, 736–741. [Google Scholar] [CrossRef]
- Liu, C.; Jiao, X.J.; He, S.; Zhao, L.C.; Zeng, X.S. A highly selective and sensitive fluorescent probe for hypochlorous acid and its lysosome-targetable biological applications. Talanta 2017, 174, 234–242. [Google Scholar] [CrossRef]
- Ren, M.G.; Li, Z.H.; Nie, J.; Wang, L.; Lin, W.Y. A photocaged fluorescent probe for imaging hypochlorous acid in lysosomes. Chem. Commun. 2018, 54, 9238–9241. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Wang, Z.Y.; Lv, Y.J.; Shen, S.L.; Zhu, Y.; Wang, J.; Zhang, Y.R.; Wang, J.M.; Ge, Y.Q.; Cao, X.Q. A fluorescent probe for the detection of HOCl in lysosomes. New J. Chem. 2018, 42, 11480–11484. [Google Scholar] [CrossRef]
- Shen, S.L.; Huang, X.Q.; Zhang, Y.Y.; Zhu, Y.; Hou, C.; Ge, Y.Q.; Cao, X.Q. Ratiometric fluorescent probe for the detection of HOCl in lysosomes based on FRET strategy. Sens. Actuators B Chem. 2018, 263, 252–257. [Google Scholar] [CrossRef]
- Zhang, P.S.; Wang, H.; Zhang, D.; Zeng, X.Y.; Zeng, R.J.; Xiao, L.H.; Tao, H.W.; Long, Y.F.; Yi, P.G.; Chen, J. Two-photon fluorescent probe for lysosome-targetable hypochlorous acid detection within living cells. Sens. Actuators B Chem. 2018, 255, 2223–2231. [Google Scholar] [CrossRef]
- Shen, S.L.; Huang, X.Q.; Jiang, H.L.; Lin, X.H.; Cao, X.Q. A rhodamine B-based probe for the detection of HOCl in lysosomes. Anal. Chim. Acta 2019, 1046, 185–191. [Google Scholar] [CrossRef]
- Shen, S.L.; Huang, X.Q.; Lin, X.H.; Cao, X.Q. A ratiometric fluorescent probe for lysosomal hypochlorous acid based on through-bond energy transfer strategy. Anal. Chim. Acta 2019, 1052, 124–130. [Google Scholar] [CrossRef]
- Xue, M.J.; Wang, H.; Chen, J.; Ren, J.Y.; Chen, S.; Yang, H.P.; Zeng, R.J.; Long, Y.F.; Zhang, P.S. Ratiometric fluorescent sensing of endogenous hypochlorous acid in lysosomes using AIE-based polymeric nanoprobe. Sens. Actuators B Chem. 2019, 282, 1–8. [Google Scholar] [CrossRef]
- Gong, Y.J.; Zhang, M.L.; Wang, B.X.; Lv, Q.; Wang, Y.; Dong, W.P. A smart approach toward rhodamine spiro-ring derivatives sensing platform for lysosome-targetable imaging applications. Sens. Actuators B Chem. 2019, 283, 239–246. [Google Scholar] [CrossRef]
- Shi, R.G.; Chen, H.; Qi, Y.P.; Huang, W.; Yin, G.; Wang, R.Y. From aggregation-induced to solution emission: A new strategy for designing ratiometric fluorescent probes and its application for in vivo HClO detection. Analyst 2019, 144, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.M.; Qian, Y. A fast-responsed lysosomal-targeted fluorescent probe based on BODIPY with low limit detection for hypochlorous acid and its application of intracellular hypochlorous acid bioimaging. Opt. Mater. 2019, 92, 53–59. [Google Scholar] [CrossRef]
- Hou, P.; Chen, S.; Liang, G.L.; Li, H.M.; Zhang, H.G. A lysosome-targeted ratiometric fluorescent probe with a large blue shift for monitoring hypochlorous acid in living cells and zebrafish. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117866. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Yu, F.; Chen, J.; Qu, Z.; Han, K. A near-infrared reversible and ratiometric fluorescent probe based on Se-BODIPY for the redox cycle mediated by hypobromous acid and hydrogen sulfide in living cells. Chem. Commun. 2013, 49, 5790–5792. [Google Scholar] [CrossRef]
- Ma, C.; Ma, M.; Zhang, Y.; Zhu, X.; Zhou, L.; Fang, R.; Liu, X.; Zhang, H. Lysosome-targeted two-photon fluorescent probe for detection of hypobromous acid in vitro and in vivo. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 48–54. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zhang, H.; Zhou, D.H.; Liu, Y.H.; Ran, X.Y.; Xiang, F.F.; Zhang, L.N.; Chen, Y.J.; Yu, X.Q.; Li, K. Migration from Lysosome to Nucleus: Monitoring Lysosomal Alkalization-Related Biological Processes with an Aminofluorene-Based Probe. Anal. Chem. 2023, 95, 7294–7302. [Google Scholar] [CrossRef]
- Zhang, T.; Hong, X.Q.; Zhi, H.T.; Hu, J.H.; Chen, W.H. Synthesis and mechanism of biological action of morpholinyl-bearing arylsquaramides as small-molecule lysosomal pH modulators. RSC Adv. 2022, 12, 22748–22759. [Google Scholar] [CrossRef]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.-N.; Li, H. Recent Development of Lysosome-Targeted Organic Fluorescent Probes for Reactive Oxygen Species. Molecules 2023, 28, 6650. https://doi.org/10.3390/molecules28186650
Nguyen V-N, Li H. Recent Development of Lysosome-Targeted Organic Fluorescent Probes for Reactive Oxygen Species. Molecules. 2023; 28(18):6650. https://doi.org/10.3390/molecules28186650
Chicago/Turabian StyleNguyen, Van-Nghia, and Haidong Li. 2023. "Recent Development of Lysosome-Targeted Organic Fluorescent Probes for Reactive Oxygen Species" Molecules 28, no. 18: 6650. https://doi.org/10.3390/molecules28186650