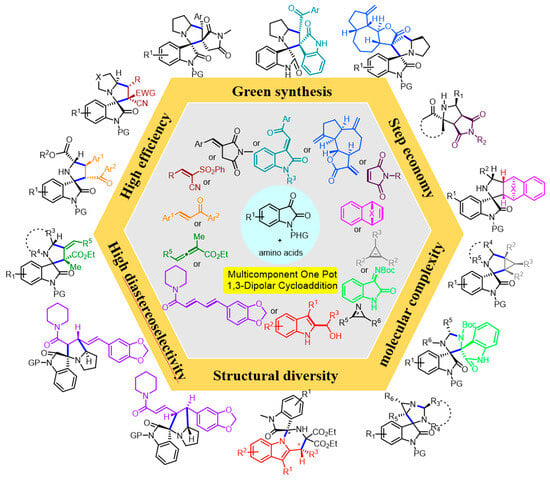
Engaging Isatins and Amino Acids in Multicomponent One-Pot 1,3-Dipolar Cycloaddition Reactions—Easy Access to Structural Diversity
Abstract
:1. Introduction
2. The Dipolarophiles Containing C=C Double Bond Location within the Linear Carbon Chains
3. Dipolarophiles Containing Exocyclic Unsaturated C=C Double Bonds
4. Dipolarophiles Containing Endocyclic Unsaturated C=C Double Bonds
5. Dipolarophiles Containing Unsaturated C=N Double Bonds
6. Other Dipolarophiles
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Coppola, G.A.; Pillitteri, S.; Van der Eycken, E.V.; You, S.-L.; Sharma, U.K. Multicomponent reactions and photo/electrochemistry join forces: Atom economy meets energy efficiency. Chem. Soc. Rev. 2022, 51, 2313–2382. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.A.D.; Rocha, R.O.; Rodrigues, M.O. Catalytic approaches to multicomponent reactions: A critical review and perspectives on the roles of catalysis. Molecules 2022, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Touré, B.B.; Hall, D.G. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 2009, 109, 4439–4486. [Google Scholar] [CrossRef]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V.A. Multicomponent reaction design in the quest for molecular complexity and diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Zaretsky, S.; Rai, V.; Yudin, A.K. Small heterocycles in multicomponent reactions. Chem. Rev. 2014, 114, 8323–8359. [Google Scholar] [CrossRef]
- Reguera, L.; Rivera, D.G. Multicomponent reaction toolbox for peptide macrocyclization and stapling. Chem. Rev. 2019, 119, 9836–9860. [Google Scholar] [CrossRef]
- Zhi, S.; Ma, X.; Zhang, W. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019, 17, 7632–7650. [Google Scholar] [CrossRef]
- Younus, H.A.; Al-Rashida, M.; Hameed, A.; Uroos, M.; Salar, U.; Rana, S.; Khan, K.M. Multicomponent reactions (MCR) in medicinal chemistry: A patent review (2010–2020). Expert. Opin. Ther. Pat. 2021, 31, 267–289. [Google Scholar] [CrossRef]
- Eftekhari-Sis, B.; Zirak, M.; Akbari, A. Arylglyoxals in synthesis of heterocyclic compounds. Chem. Rev. 2013, 113, 2958–3043. [Google Scholar] [PubMed]
- Gulevich, A.V.; Dudnik, A.S.; Chernyak, N.; Gevorgyan, V. Transition metal-mediated synthesis of monocyclic aromatic heterocycles. Chem. Rev. 2013, 113, 3084–3213. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev. 2013, 113, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Estévez, V.; Villacampa, M.; Menéndez, J.C. Recent advances in the synthesis of pyrroles by multicomponent reactions. Chem. Soc. Rev. 2014, 43, 4633–4657. [Google Scholar] [CrossRef]
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Jones, L.H.; Fadeyi, O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, K.; Zeng, C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 2018, 118, 4485–4540. [Google Scholar] [CrossRef]
- Mermer, A.; Keles, T.; Sirin, Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review. Bioorg. Chem. 2021, 114, 105076. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, X.; Wan, J.-P. Recent advances in transition metal-free annulation toward heterocycle diversity based on the C–N bond cleavage of enaminone platform. Org. Biomol. Chem. 2022, 20, 2356–2369. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-dipolar cycloadditions. Proc. Chem. Soc. 1961, 2, 357–396. [Google Scholar]
- Huisgen, R. 1,3-dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Huisgen, R. Kinetics and mechanism of 1,3-dipolar cycloadditions. Angew. Chem. Int. Ed. Engl. 1963, 2, 633–645. [Google Scholar] [CrossRef]
- Pellissier, H. Asymmetric 1,3-dipolar cycloadditions. Tetrahedron 2007, 63, 3235–3285. [Google Scholar] [CrossRef]
- Lashgari, N.; Ziarani, G.M. Synthesis of heterocyclic compounds based on isatin through 1,3-dipolar cycloaddition reactions. ARKIVOC 2012, 2012, 277–320. [Google Scholar] [CrossRef]
- Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A.M. 1,2,3-triazole in heterocyclic compounds, endowed with biological activity, through 1,3-dipolar cycloadditions. Eur. J. Org. Chem. 2014, 2014, 3289–3306. [Google Scholar] [CrossRef]
- Narayan, R.; Potowski, M.; Jia, Z.-J.; Antonchick, A.P.; Waldmann, H. Catalytic enantioselective 1,3-dipolar cycloadditions of azomethine ylides for biology-oriented synthesis. Acc. Chem. Res. 2014, 47, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Maruoka, K. Recent advances of catalytic asymmetric 1,3-dipolar cycloadditions. Chem. Rev. 2015, 115, 5366–5412. [Google Scholar] [CrossRef]
- Dommerholt, J.; Rutjes, F.P.J.T.; van Delft, F.L. Strain-promoted 1,3-dipolar cycloaddition of cycloalkynes and organic azides. Top. Curr. Chem. 2016, 374, 16. [Google Scholar] [CrossRef]
- Siadati, S.A. Beyond the alternatives that switch the mechanism of the 1,3-dipolar cycloadditions from concerted to stepwise or vice versa: A literature review. Prog. React. Kinet. Mech. 2016, 41, 331–344. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Mishra, B.B.; Mishra, K.B.; Mishra, N.; Singh, A.S.; Chen, X. Cu-catalyzed click reaction in carbohydrate chemistry. Chem. Rev. 2016, 116, 3086–3240. [Google Scholar] [CrossRef]
- Arrastia, I.; Arrieta, A.; Cossío, F.P. Application of 1,3-dipolar reactions between azomethine ylides and alkenes to the synthesis of catalysts and biologically active compounds. Eur. J. Org. Chem. 2018, 2018, 5889–5904. [Google Scholar] [CrossRef]
- Gulevskaya, A.V.; Nelina-Nemtseva, J.I. 1,3-dipolar cycloaddition reactions of azomethine ylides and alkynes. Chem. Heterocycl. Compd. 2018, 54, 1084–1107. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.-U. The huisgen reaction: Milestones of the 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef]
- Yue, G.; Liu, B. Research progress on [3 + n] (n ≥ 3) cycloaddition of 1,3-diploes. Chin. J. Org. Chem. 2020, 40, 3132–3153. [Google Scholar] [CrossRef]
- Beutick, S.E.; Vermeeren, P.; Hamlin, T.A. The 1,3-dipolar cycloaddition: From conception to quantum chemical design. Chem-Asian. J. 2022, 17, e202200553. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Guiry, P.J. Catalytic enantioselective [3 + 2] cycloaddition of N-metalated azomethine ylides. Chem.-Eur. J. 2023, 29, e202300296. [Google Scholar] [CrossRef]
- Jossang, A.; Jossang, P.; Hadi, H.A.; Sevenet, T.; Bodo, B. Horsfiline, an oxindole alkaloid from horsfieldia superba. J. Org. Chem. 1991, 56, 6527–6530. [Google Scholar] [CrossRef]
- Miyake, F.Y.; Yakushijin, K.; Horne, D.A. Preparation and synthetic applications of 2-halotryptamines: Synthesis of elacomine and isoelacomine. Org. Lett. 2004, 6, 711–713. [Google Scholar] [CrossRef]
- Song, Y.; Qu, R.; Zhu, S.; Zhang, R.; Ma, S. Rhynchophylline attenuates lps-induced pro-inflammatory responses through down-regulation of mapk/nf-κb signaling pathways in primary microglia. Phytother. Res. 2012, 26, 1528–1533. [Google Scholar] [CrossRef]
- Cui, C.-B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1996, 52, 12651–12666. [Google Scholar] [CrossRef]
- Yeap, J.S.-Y.; Tan, C.-H.; Yong, K.-T.; Lim, K.-H.; Lim, S.-H.; Low, Y.-Y.; Kam, T.-S. Macroline, talpinine, and sarpagine alkaloids from alstonia penangiana. An nmr-based method for differentiating between a. Penangiana and a. Macrophylla. Phytochemistry 2020, 176, 112391. [Google Scholar] [CrossRef]
- Shangary, S.; Qin, D.; McEachern, D.; Liu, M.; Miller, R.S.; Qiu, S.; Nikolovska-Coleska, Z.; Ding, K.; Wang, G.; Chen, J.; et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc. Natl. Acad. Sci. USA 2008, 105, 3933–3938. [Google Scholar] [CrossRef]
- Efremov, I.V.; Vajdos, F.F.; Borzilleri, K.A.; Capetta, S.; Chen, H.; Dorff, P.H.; Dutra, J.K.; Goldstein, S.W.; Mansour, M.; McColl, A.; et al. Discovery and optimization of a novel spiropyrrolidine inhibitor of β-secretase (BACE1) through fragment-based drug design. J. Med. Chem. 2012, 55, 9069–9088. [Google Scholar] [CrossRef]
- Saraswat, P.; Jeyabalan, G.; Hassan, M.Z.; Rahman, M.U.; Nyola, N.K. Review of synthesis and various biological activities of spiro heterocyclic compounds comprising oxindole and pyrrolidine moities. Synth. Commun. 2016, 46, 1643–1664. [Google Scholar] [CrossRef]
- Al-Rashood, S.T.; Hamed, A.R.; Hassan, G.S.; Alkahtani, H.M.; Almehizia, A.A.; Alharbi, A.; Al-Sanea, M.M.; Eldehna, W.M. Antitumor properties of certain spirooxindoles towards hepatocellular carcinoma endowed with antioxidant activity. J. Enzym. Inhib. Med. Chem. 2020, 35, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-M.; Qu, R.-Y.; Yang, G.-F. An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert. Opin. Drug. Dis. 2020, 15, 603–625. [Google Scholar] [CrossRef] [PubMed]
- Ball-Jones, N.R.; Badillo, J.J.; Franz, A.K. Strategies for the enantioselective synthesis of spirooxindoles. Org. Biomol. Chem. 2012, 10, 5165–5181. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wang, R. Recent advances in asymmetric organocatalytic construction of 3,3′-spirocyclic oxindoles. Adv. Synth. Catal. 2013, 355, 1023–1052. [Google Scholar] [CrossRef]
- Boddy, A.J.; Bull, J.A. Stereoselective synthesis and applications of spirocyclic oxindoles. Org. Chem. Front. 2021, 8, 1026–1084. [Google Scholar] [CrossRef]
- Ganesh, M.; Suraj, S. Expeditious entry into carbocyclic and heterocyclic spirooxindoles. Org. Biomol. Chem. 2022, 20, 5651–5693. [Google Scholar] [CrossRef]
- Borah, B.; Veeranagaiah, N.S.; Sharma, S.; Patat, M.; Prasad, M.S.; Pallepogu, R.; Chowhan, L.R. Stereoselective synthesis of CF3-containing spirocyclic-oxindoles using N-2,2,2-trifluoroethylisatin ketimines: An update. RSC Adv. 2023, 13, 7063–7075. [Google Scholar] [CrossRef]
- Nájera, C.; Sansano, J.M. Synthesis of pyrrolizidines and indolizidines by multicomponent 1,3-dipolar cycloaddition of azomethine ylides. Pure Appl. Chem. 2019, 91, 575–596. [Google Scholar] [CrossRef]
- Wang, Y.; Cobo, A.A.; Franz, A.K. Recent advances in organocatalytic asymmetric multicomponent cascade reactions for enantioselective synthesis of spirooxindoles. Org. Chem. Front. 2021, 8, 4315–4348. [Google Scholar] [CrossRef]
- Miankooshki, F.R.; Bayat, M.; Nasri, S.; Samet, N.H. 1,3-dipolar cycloaddition reactions of isatin-derived azomethine ylides for the synthesis of spirooxindole and indole-derived scaffolds: Recent developments. Mol. Divers. 2022, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.M.d.; Garden, S.J.; Pinto, A.C. The chemistry of isatins: A review from 1975 to 1999. J. Brazil. Chem. Soc. 2001, 12, 273–324. [Google Scholar] [CrossRef]
- Singh, G.S.; Desta, Z.Y. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 2012, 112, 6104–6155. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Wan, J. Recent advances in diversity oriented synthesis through isatin-based multicomponent reactions. Asian J. Org. Chem. 2013, 2, 374–386. [Google Scholar] [CrossRef]
- Gupta, A.K.; Tulsyan, S.; Bharadwaj, M.; Mehrotra, R. Systematic review on cytotoxic and anticancer potential of n-substituted isatins as novel class of compounds useful in multidrug-resistant cancer therapy: In silico and in vitro analysis. Top. Curr. Chem. 2019, 377, 15. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, N.P.; Kumar, K. Recent advancement of synthesis of isatins as a versatile pharmacophore: A review. Drug Res. 2020, 71, 115–121. [Google Scholar] [CrossRef]
- Brandão, P.; Marques, C.; Burke, A.J.; Pineiro, M. The application of isatin-based multicomponent-reactions in the quest for new bioactive and druglike molecules. Eur. J. Med. Chem. 2021, 211, 113102. [Google Scholar] [CrossRef]
- Jia, F.; Luo, N.; Xu, C.; Wu, A. Recent advances in the synthesis of benzoheterocyclic compounds involving isatins. Chin. J. Org. Chem. 2021, 41, 1527–1542. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Bayat, M. Synthesis of heterocyclic compounds based on isatins. Curr. Org. Chem. 2022, 26, 756–770. [Google Scholar] [CrossRef]
- Wang, K.-K.; Li, Y.-L.; Chen, R.-X.; Sun, A.-L.; Wang, Z.-Y.; Zhao, Y.-C.; Wang, M.-Y.; Sheng, S. Substrate-controlled regioselectivity switch in a three-component 1,3-dipolar cycloaddition reaction to access 3,3′-pyrrolidinyl-spirooxindoles derivatives. Adv. Synth. Catal. 2022, 364, 2047–2052. [Google Scholar] [CrossRef]
- Shyamsivappan, S.; Vivek, R.; Saravanan, A.; Arasakumar, T.; Subashini, G.; Suresh, T.; Shankar, R.; Mohan, P.S. Synthesis and x-ray study of dispiro 8-nitroquinolone analogues and their cytotoxic properties against human cervical cancer hela cells. MedChemComm 2019, 10, 439–449. [Google Scholar] [CrossRef]
- Zahedifar, M.; Pouramiri, B.; Ghadi, F.E.; Razavi, R.; Ghara, A.R. Unexpected regio-and stereoselective [4 + 3] cycloaddition reaction of azomethine ylides with benzylidene thiazolidinediones: Synthesis of pharmacologically active spiroindoline oxazepine derivatives and theoretical study. Mol. Divers. 2021, 25, 29–43. [Google Scholar] [CrossRef]
- Yi, Y.; Hua, Y.-Z.; Lu, H.-J.; Liu, L.-T.; Wang, M.-C. Brønsted base and lewis acid cooperatively catalyzed asymmetric exo′-selective [3 + 2] cycloaddition of trifluoromethylated azomethine ylides and methyleneindolinones. Org. Lett. 2020, 22, 2527–2531. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Han, Y.; Zeng, C.; Zhang, T.Y.; Ye, J.; Song, G. Remote regioselective organocatalytic asymmetric [3 + 2] cycloaddition of N-2,2,2-trifluoroethyl isatin ketimines with cyclic 2,4-dienones. Chin. Chem. Lett. 2020, 31, 377–380. [Google Scholar] [CrossRef]
- Zhao, J.-Q.; Zhou, S.; Yang, L.; Du, H.-Y.; You, Y.; Wang, Z.-H.; Zhou, M.-Q.; Yuan, W.-C. Catalytic asymmetric dearomative 1,3-dipolar cycloaddition of 2-nitrobenzothiophenes and isatin-derived azomethine ylides. Org. Lett. 2021, 23, 8600–8605. [Google Scholar] [CrossRef]
- Yue, G.; Wu, Y.; Dou, Z.; Chen, H.; Yin, Z.; Song, X.; He, C.; Wang, X.; Feng, J.; Zhang, Z.; et al. Synthesis of spiropyrrolidine oxindoles via ag-catalyzed stereo- and regioselective 1,3-dipolar cycloaddition of indole-based azomethine ylides with chalcones. New J. Chem. 2018, 42, 20024–20031. [Google Scholar] [CrossRef]
- Villarreal, Y.; Insuasty, B.; Abonia, R.; Ortiz, A.; Romo, P.; Quiroga, J. Three-component one-pot synthesis of new spiro[indoline-pyrrolidine] derivatives mediated by 1,3-dipolar reaction and DFT analysis. Monatshefte Fur Chem.-Chem. Mon. 2021, 152, 497–506. [Google Scholar] [CrossRef]
- de Silva, N.H.; Pyreddy, S.; Blanch, E.W.; Hügel, H.M.; Maniam, S. Microwave-assisted rapid synthesis of spirooxindole-pyrrolizidine analogues and their activity as anti-amyloidogenic agents. Bioorg. Chem. 2021, 114, 105128. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y. A facile synthesis of novel endo-selective polycyclic spiropyrrolidine oxindoles via 1,3-dipolar cycloaddition of α,γ-dialkylallenoate esters. Tetrahedron Lett. 2017, 58, 1545–1547. [Google Scholar] [CrossRef]
- Alizadeh, A.; Roosta, A.; Halvagar, M. Four-component regio- and diastereoselective synthesis of pyrrolizidines incorporating spiro-oxindole/indanedione via 1,3-dipolar cycloaddition reaction of azomethine ylides. ChemistrySelect 2019, 4, 71–74. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Ali, M.; Al-Majid, A.M.; Alshahrani, S.; Alamary, A.S.; Yousuf, S.; Choudhary, M.I. Regio-and stereoselective synthesis of a new series of spirooxindole pyrrolidine grafted thiochromene scaffolds as potential anticancer agents. Symmetry 2021, 13, 1426. [Google Scholar] [CrossRef]
- Gong, P.; Ma, Y.; Wang, X.; Yu, L.; Zhu, S. A facile and diverse synthesis of coumarin substituted spirooxindole and dispiro oxindole-pyrrolizidine/pyrrolothiazole/pyrrolidine derivatives via 1,3-dipolar cycloaddition. Tetrahedron 2021, 91, 132221. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The bioactive compound of black pepper: From isolation to medicinal formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, C.; Wang, Y.; Xu, Y.; Zhao, J.; Gao, M.; Ding, Y.; Peng, J.; Li, L. Development and evaluation of a novel drug delivery: Soluplus®/TPGS mixed micelles loaded with piperine in vitro and in vivo. Drug Dev. Ind. Pharm. 2018, 44, 1409–1416. [Google Scholar] [CrossRef]
- Pawar, K.S.; Mastud, R.N.; Pawar, S.K.; Pawar, S.S.; Bhoite, R.R.; Bhoite, R.R.; Kulkarni, M.V.; Deshpande, A.R. Oral curcumin with piperine as adjuvant therapy for the treatment of covid-19: A randomized clinical trial. Front. Pharmacol. 2021, 12, 1056. [Google Scholar] [CrossRef]
- Singh, M.; Krishnan, A.V.A.; Mandal, R.; Samanta, J.; Ravichandiran, V.; Natarajan, R.; Bharitkar, Y.P.; Hazra, A. Azomethine ylide cycloaddition: A versatile tool for preparing novel pyrrolizidino-spiro-oxindolo hybrids of the doubly conjugated alkamide piperine. Mol. Divers. 2020, 24, 627–639. [Google Scholar] [CrossRef]
- Boudriga, S.; Haddad, S.; Murugaiyah, V.; Askri, M.; Knorr, M.; Strohmann, C.; Golz, C. Three-component access to functionalized spiropyrrolidine heterocyclic scaffolds and their cholinesterase inhibitory activity. Molecules 2020, 25, 1963. [Google Scholar] [CrossRef]
- Askri, S.; Edziri, H.; Hamouda, M.B.; McHiri, C.; Gharbi, R.; El-Gawad, H.H.A.; El-Tahawy, M.M.T. Synthesis, biological evaluation, density functional calculation and molecular docking analysis of novel spiropyrrolizidines derivatives as potential anti-microbial and anti-coagulant agents. J. Mol. Struct. 2022, 1250, 131688. [Google Scholar] [CrossRef]
- Arun, Y.; Bhaskar, G.; Balachandran, C.; Ignacimuthu, S.; Perumal, P.T. Facile one-pot synthesis of novel dispirooxindole-pyrrolidine derivatives and their antimicrobial and anticancer activity against A549 human lung adenocarcinoma cancer cell line. Biorg. Med. Chem. Lett. 2013, 23, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Velikorodov, A.V.; Ionova, V.A.; Degtyarev, O.V.; Sukhenko, L.T. Synthesis and antimicrobial and antifungal activity of carbamate-functionized spiro compounds. Pharm. Chem. J. 2013, 46, 715–719. [Google Scholar] [CrossRef]
- Bhandari, S.; Sana, S.; Sridhar, B.; Shankaraiah, N. Microwave-assisted one-pot [3 + 2] cycloaddition of azomethine ylides and 3-alkenyl oxindoles: A facile approach to pyrrolidine-fused bis-spirooxindoles. ChemistrySelect 2019, 4, 1727–1730. [Google Scholar] [CrossRef]
- de Silva, N.H.; Dahdah, A.; Blanch, E.W.; Hügel, H.M.; Maniam, S. Regioselective pyrrolizidine bis-spirooxindoles as efficient anti-amyloidogenic agents. Eur. J. Med. Chem. 2022, 240, 114566. [Google Scholar] [CrossRef]
- Qian, Y.-L.; Li, B.; Xia, P.-J.; Wang, J.; Xiang, H.-Y.; Yang, H. Diastereospecific entry to pyrrolidinyl dispirooxindole skeletons via three-component 1,3-dipolar cycloadditions. Tetrahedron 2018, 74, 6821–6828. [Google Scholar] [CrossRef]
- Shahrestani, N.; Tovfighmadar, K.; Eskandari, M.; Jadidi, K.; Notash, B.; Mirzaei, P. Synthesis of highly enantioenriched bis-spirooxindole pyrrolizidine/pyrrolidines through asymmetric [3 + 2] cycloaddition reaction. Asian J. Org. Chem. 2020, 9, 822–828. [Google Scholar] [CrossRef]
- Sathi, V.; Thomas, N.V.; Deepthi, A. Stereoselective synthesis of dispiro heterocycles by [3 + 2] cycloaddition of azomethine ylides with a thiazolo[3,2-a]indole derivative. Org. Biomol. Chem. 2020, 18, 7822–7826. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Nuñez, I.S.; Castañeda-Acosta, J.; Foroozesh, M.; Fronczek, F.R.; Fischer, N.H.; Franzblau, S.G. Antimycobacterial activities of dehydrocostus lactone and its oxidation products. J. Nat. Prod. 1998, 61, 1181–1186. [Google Scholar] [CrossRef]
- Orofino Kreuger, M.R.; Grootjans, S.; Biavatti, M.W.; Vandenabeele, P.; D’Herde, K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: Focus on parthenolide. Anti-Cancer Drug. 2012, 23, 883–896. [Google Scholar] [CrossRef]
- Ghantous, A.; Sinjab, A.; Herceg, Z.; Darwiche, N. Parthenolide: From plant shoots to cancer roots. Drug Discov. Today 2013, 18, 894–905. [Google Scholar] [CrossRef]
- Cárdenas, D.M.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Synthesis of pertyolides a, b, and c: A synthetic procedure to C17-sesquiterpenoids and a study of their phytotoxic activity. J. Nat. Prod. 2021, 84, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-H.; Wang, Y.-H.; Chen, Z.-Y.; Wang, X.-R.; Zhang, W.-H.; Tian, F.-L.; Peng, L.-J.; Zhou, Y.; Liu, X.-L. Formal oxygen atom insertion as a skeletal-editing step: Rapid access natural-product-inspired bispiro[oxindole-oxazinane] hybrids. Org. Chem. Front. 2023, 10, 3307–3312. [Google Scholar] [CrossRef]
- Zhao, H.; Shao, X.; Wang, T.; Zhai, S.; Qiu, S.; Tao, C.; Wang, H.; Zhai, H. A 2-(1-methylhydrazinyl)pyridine-directed C–H functionalization/spirocyclization cascade: Facile access to spirosuccinimide derivatives. Chem. Commun. 2018, 54, 4927–4930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, T.; Qing, Z.; Zhai, H. Cobalt-catalyzed 2-(1-methylhydrazinyl)pyridine-assisted cyclization of thiophene-2-carbohydrazides with maleimides: Efficient synthesis of thiophene-fused pyridones. Chem. Commun. 2020, 56, 5524–5527. [Google Scholar] [CrossRef]
- Shen, P.; Guo, Y.; Wei, J.; Zhao, H.; Zhai, H.; Zhao, Y. Straightforward synthesis of succinimide-fused pyrrolizidines by a three-component reaction of α-diketone, amino acid, and maleimide. Synthesis 2020, 53, 1262–1270. [Google Scholar]
- Nishimura, T.; Kawamoto, T.; Sasaki, K.; Tsurumaki, E.; Hayashi, T. Rhodium-catalyzed asymmetric cyclodimerization of oxa- and azabicyclic alkenes. J. Am. Chem. Soc. 2007, 129, 1492–1493. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, W.; Yao, Y.; Li, Y.; Wang, L.; Yang, D. Copper-catalyzed cycloaddition of heterobicyclic alkenes with diaryl disulfides to synthesize dihydrobenzo[b]thiophene derivatives. J. Org. Chem. 2021, 86, 4193–4204. [Google Scholar] [CrossRef]
- Chen, W.; Yang, W.; Wu, R.; Yang, D. Water-promoted synthesis of fused bicyclic triazolines and naphthols from oxa(aza)bicyclic alkenes and transformation via a novel ring-opening/rearrangement reaction. Green Chem. 2018, 20, 2512–2518. [Google Scholar] [CrossRef]
- Yuan, K.; Zhang, T.K.; Hou, X.L. A highly efficient palladacycle catalyst for hydrophenylation of C-, N-, and O-substituted bicyclic alkenes under aerobic condition. J. Org. Chem. 2005, 70, 6085–6088. [Google Scholar] [CrossRef]
- Li, S.; Lu, Z.; Meng, L.; Wang, J. Iridium-catalyzed asymmetric addition of thiophenols to oxabenzonorbornadienes. Org. Lett. 2016, 18, 5276–5279. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, W.; Wang, C.; Gu, F.; Yang, W.; Yang, D. Radical addition of thiols to oxa(aza)bicyclic alkenes in water. Asian J. Org. Chem. 2019, 8, 506–513. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y. Copper-catalyzed hydroamination of oxa- and azabenzonorbornadienes with pyrazoles. J. Org. Chem. 2022, 87, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Khan, R.; Chen, J.; Zhang, X.; Gao, Y.; Zhou, Y.; Li, K.; Tian, Y.; Fan, B. Directing-group-controlled ring-opening addition and hydroarylation of oxa/azabenzonorbornadienes with arenes via C–H activation. Org. Lett. 2020, 22, 3339–3344. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, X.; Qi, Z.; Li, X. Palladium-catalyzed [3 + 2] annulation of aryl halides with 7-oxa- and 7-azabenzonorbornadienes via C(sp2 or sp3)–H activation. Org. Lett. 2022, 24, 8964–8968. [Google Scholar] [CrossRef]
- Kumaran, S.; Saritha, R.; Gurumurthy, P.; Parthasarathy, K. Synthesis of fused spiropyrrolidine oxindoles through 1,3-dipolar cycloaddition of azomethine ylides prepared from isatins and α-amino acids with heterobicyclic alkenes. Eur. J. Org. Chem. 2020, 2020, 2725–2729. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, H.; Zhao, Y. An efficient synthesis of oxygen-bridged spirooxindoles via microwave-promoted multicomponent reaction. Molecules 2023, 28, 3508. [Google Scholar] [CrossRef]
- Kutyashev, I.B.; Ulitko, M.V.; Barkov, A.Y.; Zimnitskiy, N.S.; Korotaev, V.Y.; Sosnovskikh, V.Y. A regio- and stereocontrolled approach to the synthesis of 4-CF3-substituted spiro[chromeno[3,4-c]pyrrolidine-oxindoles] via reversible [3 + 2] cycloaddition of azomethine ylides generated from isatins and sarcosine to 3-nitro-2-(trifluoromethyl)-2h-chromenes. New J. Chem. 2019, 43, 18495–18504. [Google Scholar]
- Cottiglia, F.; Dhanapal, B.; Sticher, O.; Heilmann, J. New chromanone acids with antibacterial activity from calophyllum brasiliense. J. Nat. Prod. 2004, 67, 537–541. [Google Scholar] [CrossRef]
- Wu, G.; Yu, G.; Kurtán, T.; Mándi, A.; Peng, J.; Mo, X.; Liu, M.; Li, H.; Sun, X.; Li, J.; et al. Versixanthones A–F, cytotoxic xanthone–chromanone dimers from the marine-derived fungus aspergillus versicolor HDN1009. J. Nat. Prod. 2015, 78, 2691–2698. [Google Scholar] [CrossRef]
- Zuo, X.; Liu, X.-L.; Wang, J.-X.; Yao, Y.-M.; Zhou, Y.-Y.; Wei, Q.-D.; Gong, Y.; Zhou, Y. Organocatalytic reaction of chromone-oxindole synthon: Access to chromanone-based spirocyclohexaneoxindoles with five adjacent stereocenters. J. Org. Chem. 2019, 84, 6679–6688. [Google Scholar] [CrossRef]
- Chen, S.; Yue, J.; Liu, X.-L.; Wang, J.-X.; Zuo, X.; Cao, Y. One-pot multicomponent construction of chromanone-fused pyrrolidinyl spirooxindole collections through a decarboxylative 1,3-dipolar [3 + 2] cycloaddition reaction. Synth. Commun. 2019, 49, 2425–2435. [Google Scholar] [CrossRef]
- Fumagalli, G.; Stanton, S.; Bower, J.F. Recent methodologies that exploit C–C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 2017, 117, 9404–9432. [Google Scholar] [CrossRef] [PubMed]
- Dian, L.; Marek, I. Asymmetric preparation of polysubstituted cyclopropanes based on direct functionalization of achiral three-membered carbocycles. Chem. Rev. 2018, 118, 8415–8434. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R. C–C bond cleavages of cyclopropenes: Operating for selective ring-opening reactions. Chem. Rev. 2021, 121, 162–226. [Google Scholar] [CrossRef] [PubMed]
- Diev, V.V.; Kostikov, R.R.; Gleiter, R.; Molchanov, A.P. Cyclopropenes in the 1,3-dipolar cycloaddition with carbonyl ylides: Experimental and theoretical evidence for the enhancement of σ-withdrawal in 3-substituted-cyclopropenes. J. Org. Chem. 2006, 71, 4066–4077. [Google Scholar] [CrossRef] [PubMed]
- Sherrill, W.M.; Rubin, M. Rhodium-catalyzed hydroformylation of cyclopropenes. J. Am. Chem. Soc. 2008, 130, 13804–13809. [Google Scholar] [CrossRef] [PubMed]
- Dian, L.; Marek, I. Pd-catalyzed enantioselective hydroalkynylation of cyclopropenes. ACS Catal. 2020, 10, 1289–1293. [Google Scholar] [CrossRef]
- Kikuchi, T.; Yasui, T.; Yamamoto, Y. Cycloaddition of cyclopropenes with alkynes via carbon–carbon double bond cleavage enabled by a ruthenium catalyst: Synthesis of cyclopentadienes and cycloheptatrienes. ACS Catal. 2023, 13, 9656–9666. [Google Scholar] [CrossRef]
- Filatov, A.S.; Knyazev, N.A.; Molchanov, A.P.; Panikorovsky, T.L.; Kostikov, R.R.; Larina, A.G.; Boitsov, V.M.; Stepakov, A.V. Synthesis of functionalized 3-spiro[cyclopropa[a]pyrrolizine]- and 3-spiro[3-azabicyclo[3.1.0]hexane]oxindoles from cyclopropenes and azomethine ylides via [3 + 2]-cycloaddition. J. Org. Chem. 2017, 82, 959–975. [Google Scholar] [CrossRef]
- Qian, Y.-L.; Xia, P.-J.; Li, J.; Zhao, Q.-L.; Xiao, J.-A.; Xiang, H.-y.; Yang, H. Diversity-driven and facile 1,3-dipolar cycloaddition to access dispirooxindole-imidazolidine scaffolds. Org. Biomol. Chem. 2017, 15, 8705–8708. [Google Scholar] [CrossRef]
- Angyal, A.; Demjén, A.; Harmat, V.; Wölfling, J.; Puskás, L.G.; Kanizsai, I. 1,3-dipolar cycloaddition of isatin-derived azomethine ylides with 2H-azirines: Stereoselective synthesis of 1,3-diazaspiro[bicyclo[3.1.0]hexane]oxindoles. J. Org. Chem. 2019, 84, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M. New perspectives in the indole ring functionalization using 2-indolylmethanols. Adv. Synth. Catal. 2020, 362, 1214–1232. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Jiang, F.; Shi, F. Organocatalytic asymmetric synthesis of indole-based chiral heterocycles: Strategies, reactions, and outreach. Acc. Chem. Res. 2020, 53, 425–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, F. Advances in catalytic asymmetric reactions using 2-indolylmethanols as platform molecules. Chin. J. Org. Chem. 2022, 42, 3351–3372. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Shi, F. Organocatalytic atroposelective synthesis of indole derivatives bearing axial chirality: Strategies and applications. Acc. Chem. Res. 2022, 55, 2562–2580. [Google Scholar] [CrossRef]
- Li, C.; Lu, H.; Sun, X.-X.; Mei, G.-J.; Shi, F. Diastereo- and enantioselective construction of spirooxindole scaffolds through a catalytic asymmetric [3 + 3] cycloaddition. Org. Biomol. Chem. 2017, 15, 4794–4797. [Google Scholar] [CrossRef]
- Bouifraden, S.; Drouot, C.; Hadrami, M.E.; Guenoun, F.; Lecointe, L.; Mai, N.; Paris, M.; Pothion, C.; Sadoune, M.; Sauvagnat, B.; et al. Some of the amino acid chemistry going on in the laboratory of amino acids, peptides and proteins. Amino Acids. 1999, 16, 345–379. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T. Unusual amino acids in medicinal chemistry. J. Med. Chem. 2016, 59, 10807–10836. [Google Scholar] [CrossRef]
- Narancic, T.; Almahboub, S.A.; O’Connor, K.E. Unnatural amino acids: Production and biotechnological potential. World. J. Microb. Biot. 2019, 35, 67. [Google Scholar] [CrossRef]
- Wei, L.; Xiao, L.; Hu, Y.; Wang, Z.; Tao, H.; Wang, C. Recent advances in metallated azomethine ylides for the synthesis of chiral unnatural α-amino acids. Chin. J. Org. Chem. 2019, 39, 2119–2130. [Google Scholar] [CrossRef]
- Gugkaeva, Z.T.; Panova, M.V.; Smol’yakov, A.F.; Medvedev, M.G.; Tsaloev, A.T.; Godovikov, I.A.; Maleev, V.I.; Larionov, V.A. Asymmetric metal-templated route to amino acids with 3-spiropyrrolidine oxindole core via a 1,3-dipolar addition of azomethine ylides to a chiral dehydroalanine Ni(II) complex. Adv. Synth. Catal. 2022, 364, 2395–2402. [Google Scholar] [CrossRef]




























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Zhao, Y. Engaging Isatins and Amino Acids in Multicomponent One-Pot 1,3-Dipolar Cycloaddition Reactions—Easy Access to Structural Diversity. Molecules 2023, 28, 6488. https://doi.org/10.3390/molecules28186488
Zhao H, Zhao Y. Engaging Isatins and Amino Acids in Multicomponent One-Pot 1,3-Dipolar Cycloaddition Reactions—Easy Access to Structural Diversity. Molecules. 2023; 28(18):6488. https://doi.org/10.3390/molecules28186488
Chicago/Turabian StyleZhao, Hua, and Yufen Zhao. 2023. "Engaging Isatins and Amino Acids in Multicomponent One-Pot 1,3-Dipolar Cycloaddition Reactions—Easy Access to Structural Diversity" Molecules 28, no. 18: 6488. https://doi.org/10.3390/molecules28186488