Phytocompounds from Amazonian Plant Species against Acute Kidney Injury: Potential Nephroprotective Effects
Abstract
:1. Introduction
2. Method
3. Secondary Metabolites and Nephroprotective Potential in Amazonian Plant Species
3.1. Classes of Compounds Present in Amazonian Plant Species
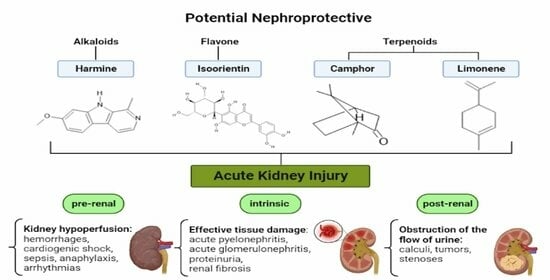
3.1.1. Alkaloids
3.1.2. Flavonoids
3.1.3. Tannins
3.1.4. Steroids
3.1.5. Terpenoids
3.2. Nefroprotective Potential of Compounds from Amazonian Plant Species
3.3. Nefroprotective Potential of Amazonian Plant Species
3.3.1. Banisteriopsis caapi (Spruce ex Griseb.) Morton
3.3.2. Peganum harmala L.
3.3.3. Passiflora edulis Sims
3.3.4. Annona muricata L.
3.3.5. Uncaria tomentosa (Willd.) DC.
3.3.6. Hymenaea courbaril L.
3.3.7. Echinodorus macrophyllus (Kunth) Micheli
3.3.8. Acmella oleracea (L.) R. K. Jansen
3.3.9. Rosmarinus officinalis L.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Carvalho Koga, R.; de Lima Teixeira dos Santos, A.V.T.; do Socorro Ferreira Rodrigues Sarquis, R.; Carvalho, J.C.T. Bauhinia guianensis Aubl., a Plant from Amazon Biome with Promising Biologically Active Properties: A Systematic Review. Pharmacogn. Rev. 2021, 15, 76–81. [Google Scholar] [CrossRef]
- Sales, P.F.; de Carvalho Rocha Koga, R.; de Souza, A.A.; do Nascimento, A.L.; Pinheiro, F.C.; Alberto, A.K.M.; da Costa, M.J.; Carvalho, J.C.T. Pharmacological Potential and Mechanisms of Action Involved in Oil Species from the Brazilian Amazon: The Case of Abelmoschus esculentus L. Moench, Euterpe Oleracea Martius and Bixa Orellana Linné. Pharmacogn. Rev 2023, 17, 24–42. [Google Scholar] [CrossRef]
- Méril-Mamert, V.; Ponce-Mora, A.; Sylvestre, M.; Lawrence, G.; Bejarano, E.; Cebrián-Torrejón, G. Antidiabetic Potential of Plants from the Caribbean Basin. Plants 2022, 11, 1360. [Google Scholar] [CrossRef]
- Rates, S.M.K. Plants as Source of Drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Da Silva, H.R.; de Assis, D.D.C.; Ariadna, L.P.; Hady, K.; Jesus, R.R.A.; José, C.T.C. Euterpe Oleracea Mart. (Aai): An Old Known Plant with a New Perspective. Afr. J. Pharm. Pharmacol. 2016, 10, 995–1006. [Google Scholar] [CrossRef]
- Carvalho, J. Fitoterápicos Anti-Inflamatórios, 2nd ed.; Pharmabooks: São Paulo, Brazil, 2017; ISBN 139788589731805. [Google Scholar]
- Carvalho, J.C.T.; Perazzo, F.F.; Machado, L.; Bereau, D. Biologic Activity and Biotechnological Development of Natural Products. BioMed Res. Int. 2013, 2013, 971745. [Google Scholar] [CrossRef]
- Isgut, M.; Rao, M.; Yang, C.; Subrahmanyam, V.; Rida, P.C.G.; Aneja, R. Application of Combination High-Throughput Phenotypic Screening and Target Identification Methods for the Discovery of Natural Product-Based Combination Drugs. Med. Res. Rev. 2018, 38, 504–524. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Kang, H.G.; Lee, H.K.; Cho, K.B.; Park, S.I. A Review of Natural Products for Prevention of Acute Kidney Injury. Medicina 2021, 57, 1266. [Google Scholar] [CrossRef]
- Neyra, J.A.; Chawla, L.S. Acute Kidney Disease to Chronic Kidney Disease. Crit. Care Clin. 2021, 37, 453–474. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute Kidney Injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef]
- Macedo, E.; Bouchard, J.; Soroko, S.H.; Chertow, G.M.; Himmelfarb, J.; Ikizker, T.A.; Paganini, E.P.; Mehta, R.L. Fluid Accumulation, Recognition and Staging of Acute Kidney Injury in Critically-Ill Patients. Crit. Care 2010, 14, R82. [Google Scholar] [CrossRef]
- Lewington, A.J.P.; Cerdá, J.; Mehta, R.L. Raising Awareness of Acute Kidney Injury: A Global Perspective of a Silent Killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Pamunuwa, G.; Karunaratne, D.N.; Waisundara, V.Y. Antidiabetic Properties, Bioactive Constituents, and Other Therapeutic Effects of Scoparia dulcis. Evid.-Based Complement. Altern. Med. 2016, 2016, 8243215. [Google Scholar] [CrossRef]
- Yang, L.; Stöckigt, J. Trends for Diverse Production Strategies of Plant Medicinal Alkaloids. Nat. Prod. Rep. 2010, 27, 1469. [Google Scholar] [CrossRef]
- Zenk, M.H.; Juenger, M. Evolution and Current Status of the Phytochemistry of Nitrogenous Compounds. Phytochemistry 2007, 68, 2757–2772. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B. Roles of Alkaloids from Medicinal Plants in the Management of Diabetes Mellitus. J. Chem. 2021, 2021, 2691525. [Google Scholar] [CrossRef]
- Putra, I.M.W.A.; Fakhrudin, N.; Nurrochmad, A.; Wahyuono, S. A Review of Medicinal Plants with Renoprotective Activity in Diabetic Nephropathy Animal Models. Life 2023, 13, 560. [Google Scholar] [CrossRef]
- Gangasani, J.K.; Pemmaraju, D.B.; Murthy, U.S.N.; Rengan, A.K.; Naidu, V.G.M. Chemistry of Herbal Biomolecules. In Herbal Biomolecules in Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 63–79. [Google Scholar]
- Sawant, M.; Isaac, J.C.; Narayanan, S. Analgesic Studies on Total Alkaloids and Alcohol Extracts of Eclipta Alba (Linn.) Hassk. Phytother. Res. 2004, 18, 111–113. [Google Scholar] [CrossRef]
- Morales-García, J.A.; de la Fuente Revenga, M.; Alonso-Gil, S.; Rodríguez-Franco, M.I.; Feilding, A.; Perez-Castillo, A.; Riba, J. The Alkaloids of Banisteriopsis Caapi, the Plant Source of the Amazonian Hallucinogen Ayahuasca, Stimulate Adult Neurogenesis in Vitro. Sci. Rep. 2017, 7, 5309. [Google Scholar] [CrossRef]
- Batista, L.L.; do Nascimento, L.C.; Guimarães, G.F.; Matias Pereira, A.C.; Koga, R.d.C.R.; Teixeira dos Santos, A.V.T.d.L.; Fernandes, C.P.; Teixeira, T.A.; Hu, Y.; Hu, X.; et al. A Review of Medicinal Plants Traditionally Used to Treat Male Sexual Dysfunctions—The Overlooked Potential of Acmella oleracea (L.) R.K. Jansen. Pharmacogn. Rev. 2021, 15, 1–11. [Google Scholar] [CrossRef]
- Rasouli, H.; Yarani, R.; Pociot, F.; Popović-Djordjević, J. Anti-Diabetic Potential of Plant Alkaloids: Revisiting Current Findings and Future Perspectives. Pharmacol. Res. 2020, 155, 104723. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, M.; Roshankhah, S.; Motavalian, V.; Jalili, C. Effect of Harmine on Nicotine-Induced Kidney Dysfunction in Male Mice. Int. J. Prev. Med. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Lou, D.; Zhou, L.; Wang, J.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Natural Products: Potential Treatments for Cisplatin-Induced Nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Boonen, J.; Chauhan, N.S.; Thakur, M.; de Spiegeleer, B.; Dixit, V.K. Spilanthes acmella Ethanolic Flower Extract: LC–MS Alkylamide Profiling and Its Effects on Sexual Behavior in Male Rats. Phytomedicine 2011, 18, 1161–1169. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of Dietary Flavonoids: Nomenclature, Occurrence and Intake. J. Nutr. 2003, 133, S3248–S3254. [Google Scholar] [CrossRef] [PubMed]
- Banjarnahor, S.D.S.; Artanti, N. Antioxidant Properties of Flavonoids. Med. J. Indones. 2015, 23, 239–244. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; Van Den Berg, D.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.F.; Bast, A. Structural Aspects of Antioxidant Activity of Flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xu, B. Antidiabetic Properties of Dietary Flavonoids: A Cellular Mechanism Review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as Antiobesity Agents: A Review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, M.K.; Veerapur, V.; Nayak, Y.; Mudgal, P.P.; Mathew, G. Antidiabetic, Antihyperlipidemic and Antioxidant Effects of the Flavonoids. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 143–161. [Google Scholar]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and New Aspects of a Class of Natural Therapeutic Drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Lu, X.; Liu, T.; Chen, K.; Xia, Y.; Dai, W.; Xu, S.; Xu, L.; Wang, F.; Wu, L.; Li, J.; et al. Isorhamnetin: A Hepatoprotective Flavonoid Inhibits Apoptosis and Autophagy via P38/PPAR-α Pathway in Mice. Biomed. Pharmacother. 2018, 103, 800–811. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P.E. The Neuroprotective Potential of Flavonoids: A Multiplicity of Effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Vargas, F.; Romecín, P.; García-Guillén, A.I.; Wangesteen, R.; Vargas-Tendero, P.; Paredes, M.D.; Atucha, N.M.; García-Estañ, J. Flavonoids in Kidney Health and Disease. Front. Physiol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Silva, W.T.; dos Santos, J.G.; Watanabe, M.; Vattimo, M.d.F.F. Efeito Renoprotetor Dos Flavonoides Do Vinho Na Nefrotoxicidade Do Imunossupressor Tacrolimus. Acta Paul. Enferm. 2011, 24, 388–392. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential Toxicity of Flavonoids and Other Dietary Phenolics: Significance for Their Chemopreventive and Anticancer Properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, Antifungal, and Antiviral Activities of Some Flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef]
- Kumar, G.; Banu, G.S.; Pappa, P.V.; Sundararajan, M.; Pandian, M.R. Hepatoprotective Activity of Trianthema portulacastrum L. against Paracetamol and Thioacetamide Intoxication in Albino Rats. J. Ethnopharmacol. 2004, 92, 37–40. [Google Scholar] [CrossRef]
- Peng, P.; Zou, J.; Zhong, B.; Zhang, G.; Zou, X.; Xie, T. Protective Effects and Mechanisms of Flavonoids in Renal Ischemia-Reperfusion Injury. Pharmacology 2023, 108, 27–36. [Google Scholar] [CrossRef]
- Korkmaz, A.; Kolankaya, D. Protective Effect of Rutin on the Ischemia/Reperfusion Induced Damage in Rat Kidney. J. Surg. Res. 2010, 164, 309–315. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Du, Y.; Chen, Z.; Guo, J.; Weng, X.; Wang, X.; Wang, M.; Chen, D.; Liu, X. Effects of Apigenin Pretreatment against Renal Ischemia/Reperfusion Injury via Activation of the JAK2/STAT3 Pathway. Biomed. Pharmacother. 2017, 95, 1799–1808. [Google Scholar] [CrossRef]
- Liu, M.; Grigoryev, D.N.; Crow, M.T.; Haas, M.; Yamamoto, M.; Reddy, S.P.; Rabb, H. Transcription Factor Nrf2 Is Protective during Ischemic and Nephrotoxic Acute Kidney Injury in Mice. Kidney Int. 2009, 76, 277–285. [Google Scholar] [CrossRef]
- Viñas, J.L.; Sola, A.; Genescà, M.; Alfaro, V.; Pí, F.; Hotter, G. NO and NOS Isoforms in the Development of Apoptosis in Renal Ischemia/Reperfusion. Free Radic Biol. Med. 2006, 40, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Okusa, M.D. The Inflammatory Cascade in Acute Ischemic Renal Failure. Nephron 2002, 90, 133–138. [Google Scholar] [CrossRef]
- Wu, H.; Chen, G.; Wyburn, K.R.; Yin, J.; Bertolino, P.; Eris, J.M.; Alexander, S.I.; Sharland, A.F.; Chadban, S.J. TLR4 Activation Mediates Kidney Ischemia/Reperfusion Injury. J. Clin. Investig. 2007, 117, 2847–2859. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Murugesan, A.G. Hypolipidaemic Activity of Helicteres isora L. Bark Extracts in Streptozotocin Induced Diabetic Rats. J. Ethnopharmacol. 2008, 116, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Constabel, C.P. Tannins in Plant–Herbivore Interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- White, T. Tannins—Their Occurrence and Significance. J. Sci. Food Agric. 1957, 8, 377–385. [Google Scholar] [CrossRef]
- Bacelo, H.A.M.; Santos, S.C.R.; Botelho, C.M.S. Tannin-Based Biosorbents for Environmental Applications—A Review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y. Tannins from Canarium Album with Potent Antioxidant Activity. J. Zhejiang Univ. Sci. B 2008, 9, 407–415. [Google Scholar] [CrossRef]
- Liu, J.-B.; Ding, Y.-S.; Zhang, Y.; Chen, J.-B.; Cui, B.-S.; Bai, J.-Y.; Lin, M.-B.; Hou, Q.; Zhang, P.-C.; Li, S. Anti-Inflammatory Hydrolyzable Tannins from Myricaria bracteata. J. Nat. Prod. 2015, 78, 1015–1025. [Google Scholar] [CrossRef]
- Tamokou, J.D.D.; Mbaveng, A.T.; Kuete, V. Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar]
- Okuda, T. Systematics and Health Effects of Chemically Distinct Tannins in Medicinal Plants. Phytochemistry 2005, 66, 2012–2031. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Eddouks, M. The Promising Role of Plant Tannins as Bioactive Antidiabetic Agents. Curr. Med. Chem. 2019, 26, 4852–4884. [Google Scholar] [CrossRef]
- Wei, X.; Luo, C.; He, Y.; Huang, H.; Ran, F.; Liao, W.; Tan, P.; Fan, S.; Cheng, Y.; Zhang, D.; et al. Hepatoprotective Effects of Different Extracts From Triphala Against CCl4-Induced Acute Liver Injury in Mice. Front. Pharmacol. 2021, 12, 664607. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Oladele, J.O.; Oladele, O.T.; Ademiluyi, A.O.; Oyeleke, O.M.; Awosanya, O.O.; Oyewole, O.I. Chaya (Jatropha tanjorensis) Leafs Protect against Sodium Benzoate Mediated Renal Dysfunction and Hepatic Damage in Rats. Clin. Phytoscience 2020, 6, 13. [Google Scholar] [CrossRef]
- Barros, M.E.; Schor, N.; Boim, M.A. Effects of an Aqueous Extract from Phyllantus Niruri on Calcium Oxalate Crystallization in Vitro. Urol. Res. 2003, 30, 374–379. [Google Scholar] [CrossRef]
- Coordenação da Farmacopeia Brasileira. Farmacopeia Brasileira, 6th ed.; Distrito Federal—ANVISA: Brasilia, Brazil, 2019.
- Kolekar, S.M.; Jain, B.U.; Kondawarkar, M.S. A Review on Steroids and Terpenoids (Stereochemistry, Structural Elucidation, Isolation of Steroids and Terpenoids). Res. J. Pharm. Dos. Forms Technol. 2019, 11, 126. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and Their Derivatives: Structural Diversity, Distribution, Metabolism, Analysis, and Health-Promoting Uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Asami, T. Brassinosteroid Biosynthesis Inhibitors. Trends Plant Sci. 1999, 4, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Jovanović-Šanta, S.S.; Petri, E.T.; Klisurić, O.R.; Szécsi, M.; Kovačević, R.; Petrović, J.A. Antihormonal Potential of Selected D-Homo and D-Seco Estratriene Derivatives. Steroids 2015, 97, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.R.; Bajaj, V.K.; Shekhawat, P.S.; Singh, K. Screening of Potential Male Contraceptive Drugs from Natural Resources: An Overview. Int. J. Pharm. Sci. Res. 2013, 4, 1654–1668. [Google Scholar]
- Thao, N.P.; Luyen, B.T.T.; Kim, E.J.; Kang, J., II; Kang, H.K.; Cuong, N.X.; Nam, N.H.; Van Kiem, P.; Van Minh, C.; Kim, Y.H. Steroidal Constituents from the Edible Sea Urchin Diadema Savignyi Michelin Induce Apoptosis in Human Cancer Cells. J. Med. Food 2015, 18, 45–53. [Google Scholar] [CrossRef]
- Rattanasopa, C.; Phungphong, S.; Wattanapermpool, J.; Bupha-Intr, T. Significant Role of Estrogen in Maintaining Cardiac Mitochondrial Functions. J. Steroid. Biochem. Mol. Biol. 2015, 147, 1–9. [Google Scholar] [CrossRef]
- Sultan, A. Steroids: A Diverse Class of Secondary Metabolites. Med. Chem. 2015, 5, 7. [Google Scholar] [CrossRef]
- Aav, R.; Kanger, T.; Pehk, T.; Lopp, M. Unexpected Reactivity of Ethyl 2-(Diethylphosphono)Propionate Toward 2,2-Disubstituted-1,3-Cyclopentanediones. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 1739–1748. [Google Scholar] [CrossRef]
- Santo, B.L.S.d.E.; Santana, L.F.; Kato Junior, W.H.; Araújo, F.d.O.d.; Tatara, M.B.; Croda, J.; Bogo, D.; Freitas, K.d.C.; Guimarães, R.d.C.A.; Hiane, P.A.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules 2020, 25, 4513. [Google Scholar] [CrossRef] [PubMed]
- Mahipal, P.; Pawar, R.S. Nephroprotective Effect of Murraya Koenigii on Cyclophosphamide Induced Nephrotoxicity in Rats. Asian Pac. J. Trop. Med. 2017, 10, 808–812. [Google Scholar] [CrossRef]
- Dennis, J.; Witting, P. Protective Role for Antioxidants in Acute Kidney Disease. Nutrients 2017, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes. In Biochemistry of Plant Secondary Metabolism; Wiley-Blackwell: Oxford, UK, 2010; pp. 258–303. [Google Scholar]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites). In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; American Society of Plant Physiologists: Rockville, MA, USA, 2000; Volume 24, pp. 1250–1319. [Google Scholar]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 233–266. [Google Scholar]
- Jahangeer, M.; Fatima, R.; Ashiq, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Therapeutic and Biomedical Potentialities of Terpenoids—A Review. J. Pure Appl. Microbiol. 2021, 15, 471–483. [Google Scholar] [CrossRef]
- Orban, J.-C.; Quintard, H.; Cassuto, E.; Jambou, P.; Samat-Long, C.; Ichai, C. Effect of N-Acetylcysteine Pretreatment of Deceased Organ Donors on Renal Allograft Function. Transplantation 2015, 99, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.-K.; Sung, S.-A.; Cho, W.-Y.; Go, K.-J.; Kim, H.-K. Macrophages Contribute to the Initiation of Ischaemic Acute Renal Failure in Rats. Nephrol. Dial. Transplant. 2006, 21, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.D.; van Goor, H.; Eddy, A.A. Macrophage Diversity in Renal Injury and Repair. J. Clin. Investig. 2008, 118, 3522–3530. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Zuk, A. Ischemic Acute Renal Failure: An Inflammatory Disease? Kidney Int. 2004, 66, 480–485. [Google Scholar] [CrossRef]
- Jablonska, J.; Wu, C.-F.; Andzinski, L.; Leschner, S.; Weiss, S. CXCR2-Mediated Tumor-Associated Neutrophil Recruitment Is Regulated by IFN-β. Int. J. Cancer 2014, 134, 1346–1358. [Google Scholar] [CrossRef]
- Glennon-Alty, L.; Hackett, A.P.; Chapman, E.A.; Wright, H.L. Neutrophils and Redox Stress in the Pathogenesis of Autoimmune Disease. Free Radic. Biol. Med. 2018, 125, 25–35. [Google Scholar] [CrossRef]
- Issa, R.; Xie, S.; Lee, K.-Y.; Stanbridge, R.D.; Bhavsar, P.; Sukkar, M.B.; Chung, K.F. GRO-α Regulation in Airway Smooth Muscle by IL-1β and TNF-α: Role of NF-ΚB and MAP Kinases. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 291, L66–L74. [Google Scholar] [CrossRef]
- Korbecki, J.; Kupnicka, P.; Chlubek, M.; Gorący, J.; Gutowska, I.; Baranowska-Bosiacka, I. CXCR2 Receptor: Regulation of Expression, Signal Transduction, and Involvement in Cancer. Int. J. Mol. Sci. 2022, 23, 2168. [Google Scholar] [CrossRef]
- Gay, N.J.; Symmons, M.F.; Gangloff, M.; Bryant, C.E. Assembly and Localization of Toll-like Receptor Signalling Complexes. Nat. Rev. Immunol. 2014, 14, 546–558. [Google Scholar] [CrossRef]
- Sollinger, D.; Eißler, R.; Lorenz, S.; Strand, S.; Chmielewski, S.; Aoqui, C.; Schmaderer, C.; Bluyssen, H.; Zicha, J.; Witzke, O.; et al. Damage-Associated Molecular Pattern Activated Toll-like Receptor 4 Signalling Modulates Blood Pressure in l-NAME-Induced Hypertension. Cardiovasc. Res. 2014, 101, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, G.F.; Dos Santos, R.A.; Oliveira, M.A.; Giachini, F.R.; Akamine, E.H.; Tostes, R.C.; Fortes, Z.B.; Webb, R.C.; Carvalho, M.H.C. Toll-like Receptor 4 Contributes to Blood Pressure Regulation and Vascular Contraction in Spontaneously Hypertensive Rats. Clin. Sci. 2012, 122, 535–543. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, S.-H.; Kim, Y.-G.; Kim, S.-Y.; Seo, J.-W.; Choi, Y.-W.; Kim, D.-J.; Jeong, K.-H.; Lee, T.-W.; Ihm, C.-G.; et al. Hyperuricemia-Induced NLRP3 Activation of Macrophages Contributes to the Progression of Diabetic Nephropathy. Am. J. Physiol.-Ren. Physiol. 2015, 308, F993–F1003. [Google Scholar] [CrossRef]
- Anders, H.-J.; Schaefer, L. Beyond Tissue Injury—Damage-Associated Molecular Patterns, Toll-Like Receptors, and Inflammasomes Also Drive Regeneration and Fibrosis. J. Am. Soc. Nephrol. 2014, 25, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting Edge: NF-ΚB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Zhang, B.; Ramesh, G.; Norbury, C.C.; Reeves, W.B. Cisplatin-Induced Nephrotoxicity Is Mediated by Tumor Necrosis Factor-α Produced by Renal Parenchymal Cells. Kidney Int. 2007, 72, 37–44. [Google Scholar] [CrossRef]
- Peres, L.A.B.; da Cunha, A.D., Jr. Acute Nephrotoxicity of Cisplatin: Molecular Mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A Novel Biomarker for Human Renal Proximal Tubule Injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Regueira, T.; Andresen, M.; Mercado, M.; Downey, P. Fisiopatología de La Insuficiencia Renal Aguda Durante La Sepsis. Med. Intensiva 2011, 35, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.P.D.; Gomes, A.S.; Lima, F.J.B.; Brito, T.S.; Soares, P.M.G.; Pinho, J.P.M.; Silva, C.S.; Santos, A.A.; Souza, M.H.L.P.; Magalhães, P.J.C. Inhaled 1,8-Cineole Reduces Inflammatory Parameters in Airways of Ovalbumin-Challenged Guinea Pigs. Basic Clin. Pharmacol. Toxicol. 2011, 108, 34–39. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, N.A.G.; Carvalho Rodrigues, M.A.; Martins, N.M.; dos Santos, A.C. Cisplatin-Induced Nephrotoxicity and Targets of Nephroprotection: An Update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. P38 MAP Kinase Inhibition Ameliorates Cisplatin Nephrotoxicity in Mice. Am. J. Physiol. Renal Physiol. 2005, 289, F166–F174. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.; Ambriz-Perez, D.; Heredia, J. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Ye, M.; Wang, Q.; Zhang, W.; Li, Z.; Wang, Y.; Hu, R. Oroxylin A Exerts Anti-Inflammatory Activity on Lipopolysaccharide-Induced Mouse Macrophage via Nrf2/ARE Activation. Biochem. Cell Biol. 2014, 92, 337–348. [Google Scholar] [CrossRef]
- Chen, X.-L.; Dodd, G.; Thomas, S.; Zhang, X.; Wasserman, M.A.; Rovin, B.H.; Kunsch, C. Activation of Nrf2/ARE Pathway Protects Endothelial Cells from Oxidant Injury and Inhibits Inflammatory Gene Expression. Am. J. Physiol. -Heart Circ. Physiol. 2006, 290, H1862–H1870. [Google Scholar] [CrossRef]
- Yu, J.S.; Lim, S.H.; Lee, S.R.; Choi, C.-I.; Kim, K.H. Antioxidant and Anti-Inflammatory Effects of White Mulberry (Morus alba L.) Fruits on Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Molecules 2021, 26, 920. [Google Scholar] [CrossRef] [PubMed]
- Vattimo, M.d.F.F.; da Silva, N.O. Uncária Tomentosa e a Lesão Renal Aguda Isquêmica Em Ratos. Rev. Esc. Enferm. USP 2011, 45, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.V. Tropicos.Org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- Schwarz, M.J.; Houghton, P.J.; Rose, S.; Jenner, P.; Lees, A.D. Activities of Extract and Constituents of Banisteriopsis Caapi Relevant to Parkinsonism. Pharmacol. Biochem. Behav. 2003, 75, 627–633. [Google Scholar] [CrossRef]
- Pic-Taylor, A.; da Motta, L.G.; de Morais, J.A.; Junior, W.M.; Santos, A.d.F.A.; Campos, L.A.; Mortari, M.R.; von Zuben, M.V.; Caldas, E.D. Behavioural and Neurotoxic Effects of Ayahuasca Infusion (Banisteriopsis Caapi and Psychotria Viridis) in Female Wistar Rat. Behav. Process. 2015, 118, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, V.; Rahman, M.d.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.-H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Joshi, V.C.; Muhammad, I. Banisteriopsis Caapi, a Unique Combination of MAO Inhibitory and Antioxidative Constituents for the Activities Relevant to Neurodegenerative Disorders and Parkinson’s Disease. J. Ethnopharmacol. 2010, 127, 357–367. [Google Scholar] [CrossRef]
- Santos, B.W.L.; Moreira, D.C.; Borges, T.K.d.S.; Caldas, E.D. Components of Banisteriopsis Caapi, a Plant Used in the Preparation of the Psychoactive Ayahuasca, Induce Anti-Inflammatory Effects in Microglial Cells. Molecules 2022, 27, 2500. [Google Scholar] [CrossRef]
- Ghanbari, A.; Jalili, C.; Salahshoor, M.; Javanmardy, S.; Ravankhah, S.; Akhshi, N. Harmine Mitigates Cisplatin-Induced Renal Injury in Male Mice through Antioxidant, Anti-Inflammatory, and Anti-Apoptosis Effects. Res. Pharm. Sci. 2022, 17, 417. [Google Scholar] [CrossRef] [PubMed]
- Araújo Galdino, O.; de Souza Gomes, I.; Ferreira de Almeida Júnior, R.; Conceição Ferreira de Carvalho, M.I.; Abreu, B.J.; Abbott Galvão Ururahy, M.; Cabral, B.; Zucolotto Langassner, S.M.; Costa de Souza, K.S.; Augusto de Rezende, A. The Nephroprotective Action of Passiflora edulis in Streptozotocin-Induced Diabetes. Sci. Rep. 2022, 12, 17546. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, Y.; Bai, M.; Li, H.; Li, L. Anxiolytic and Sedative Activities of Passiflora edulis f. Flavicarpa. J. Ethnopharmacol. 2010, 128, 148–153. [Google Scholar] [CrossRef]
- Sena, L.M.; Zucolotto, S.M.; Reginatto, F.H.; Schenkel, E.P.; De Lima, T.C.M. Neuropharmacological Activity of the Pericarp of Passiflora edulis Flavicarpa Degener: Putative Involvement of C -Glycosylflavonoids. Exp. Biol. Med. 2009, 234, 967–975. [Google Scholar] [CrossRef]
- Doungue, H.T.; Kengne, A.P.N.; Kuate, D. Neuroprotective Effect and Antioxidant Activity of Passiflora edulis Fruit Flavonoid Fraction, Aqueous Extract, and Juice in Aluminum Chloride-Induced Alzheimer’s Disease Rats. Nutrire 2018, 43, 23. [Google Scholar] [CrossRef]
- Taïwe, G.S.; Kuete, V. Passiflora edulis. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 513–526. [Google Scholar]
- Chan, W.-J.J.; McLachlan, A.J.; Hanrahan, J.R.; Harnett, J.E. The Safety and Tolerability of Annona muricata Leaf Extract: A Systematic Review. J. Pharm. Pharmacol. 2019, 72, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gyesi, J.N.; Opoku, R.; Borquaye, L.S. Chemical Composition, Total Phenolic Content, and Antioxidant Activities of the Essential Oils of the Leaves and Fruit Pulp of Annona muricata L. (Soursop) from Ghana. Biochem. Res. Int. 2019, 2019, 4164576. [Google Scholar] [CrossRef]
- Adewole, S.; Caxton-Martins, E. Morphological Changes and Hypoglycemic Effects of Annona muricata Linn. (Annonaceae) Leaf Aqueous Extract on Pancreatic β-Cells of Streptozotocin-Treated Diabetic Rats. Afr. J. Biomed. Res. 2009, 9. [Google Scholar] [CrossRef]
- Pilarski, R.; Zieliński, H.; Ciesiołka, D.; Gulewicz, K. Antioxidant Activity of Ethanolic and Aqueous Extracts of Uncaria tomentosa (Willd.) DC. J. Ethnopharmacol. 2006, 104, 18–23. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Magdy Beshbishy, A.; Wasef, L.; Elewa, Y.H.A.; Abd El-Hack, M.E.; Taha, A.E.; Al-Sagheer, A.A.; Devkota, H.P.; Tufarelli, V. Uncaria tomentosa (Willd. Ex Schult.) DC.: A Review on Chemical Constituents and Biological Activities. Appl. Sci. 2020, 10, 2668. [Google Scholar] [CrossRef]
- Khoo, S.F.; Oehlschlager, A.C.; Ourisson, G. Structure and Stereochemistry of the Diterpenes of Hymenaea courbaril (Caesalpinioideae) Seed Pod Resin. Tetrahedron 1973, 29, 3379–3388. [Google Scholar] [CrossRef]
- Spera, K.D.; Figueiredo, P.A.; Santos, P.C.; Barbosa, F.C.; Alves, C.P.; Dokkedal, A.L.; Saldanha, L.L.; Silva, L.P.; Figueiredo, C.R.; Ferreira, P.C.; et al. Genotoxicity, Anti-Melanoma and Antioxidant Activities of Hymenaea courbaril L. Seed Extract. An. Acad. Bras. Cienc. 2019, 91, e20180446. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.; Miranda, R.R.S.; Ferraz, V.P.; Pereira, M.T.; de Siqueira, E.P.; Alcântara, A.F.C. Changes in the Essential Oil Composition of Leaves of Echinodorus macrophyllus Exposed to γ-Radiation. Rev. Bras. Farmacogn. 2013, 23, 600–607. [Google Scholar] [CrossRef]
- Gasparotto, F.; Lívero, F.; Palozi, R.; Ames, M.; Nunes, B.; Donadel, G.; Ribeiro, R.; Lourenço, E.; Kassuya, C.; Junior, A. Heart-Protective Effects of Echinodorus grandiflorus in Rabbits That Are Fed a High-Cholesterol Diet. Planta Med. 2018, 84, 1271–1279. [Google Scholar] [CrossRef]
- Fernandes, D.C.; Martins, B.P.M.P.; Medeiros, D.L.; Santos, S.V.; Gayer, C.R.; Velozo, L.S.; Coelho, M.G. Antinociceptive and Anti-Inflammatory Activities of the Hexanic Extract of “Echinodorus macrophyllus” (Kunth) Micheli in Mice. Braz. J. Health Biomed. Sci. 2019, 18, 25–32. [Google Scholar]
- Spinozzi, E.; Ferrati, M.; Baldassarri, C.; Cappellacci, L.; Marmugi, M.; Caselli, A.; Benelli, G.; Maggi, F.; Petrelli, R. A Review of the Chemistry and Biological Activities of Acmella oleracea (“Jambù”, Asteraceae), with a View to the Development of Bioinsecticides and Acaricides. Plants 2022, 11, 2721. [Google Scholar] [CrossRef] [PubMed]
- Abeysiri, G.R.P.I.; Dharmadasa, R.M.; Abeysinghe, D.C.; Samarasinghe, K. Screening of Phytochemical, Physico-Chemical and Bioactivity of Different Parts of Acmella oleraceae Murr. (Asteraceae), a Natural Remedy for Toothache. Ind. Crops Prod. 2013, 50, 852–856. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive Metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–867. [Google Scholar] [CrossRef]
- Batista, L.L.; Koga, R.d.C.R.; Teixeira, A.V.T.d.L.; Teixeira, T.A.; de Melo, E.L.; Carvalho, J.C.T. Clinical Safety of a Pharmaceutical Formulation Containing an Extract of Acmella oleracea (L.) in Patients With Premature Ejaculation: A Pilot Study. Am. J. Men’s Health 2023, 17, 155798832311678. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.; Dias Ribeiro da Silva, I.; Duarte Viana, M.; Costa de Melo, N.; Sánchez-Ortiz, B.; Maia Rebelo de Oliveira, M.; Ramos Barbosa, W.; Maciel Ferreira, I.; Tavares Carvalho, J. Acute Toxicity of the Hydroethanolic Extract of the Flowers of Acmella oleracea L. in Zebrafish (Danio rerio): Behavioral and Histopathological Studies. Pharmaceuticals 2019, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis Essential Oil: A Review of Its Phytochemistry, Anti-Inflammatory Activity, and Mechanisms of Action Involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef]
- Linnaeus, C.V. Tropicos.Org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Improving Health Benefits with Considering Traditional and Modern Health Benefits of Peganum Harmala. Clin. Phytosci. 2021, 7, 18. [Google Scholar] [CrossRef]
- Niroumand, M.C.; Farzaei, M.H.; Amin, G. Medicinal Properties of Peganum Harmala L. in Traditional Iranian Medicine and Modern Phytotherapy: A Review. J. Tradit. Chin. Med. 2015, 35, 104–109. [Google Scholar] [CrossRef]
- Niu, X.; Yao, Q.; Li, W.; Zang, L.; Li, W.; Zhao, J.; Liu, F.; Zhi, W. Harmine Mitigates LPS-Induced Acute Kidney Injury through Inhibition of the TLR4-NF-ΚB/NLRP3 Inflammasome Signalling Pathway in Mice. Eur. J. Pharmacol. 2019, 849, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Sims, J. Tropicos.Org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- De Melo, N.F.; Cervi, A.C.; Guerra, M. Karyology and Cytotaxonomy of the Genus Passiflora L. (Passifloraceae). Plant Syst. Evol. 2001, 226, 69–84. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Zhou, T.; Wang, F.; Zhou, Y.; Li, Y.; Zhang, J.-J.; Zheng, J.; Xu, D.-P.; Li, H.-B. The Effects of Syzygium samarangense, Passiflora edulis and Solanum muricatum on Alcohol-Induced Liver Injury. Int. J. Mol. Sci. 2016, 17, 1616. [Google Scholar] [CrossRef] [PubMed]
- Salles, B.C.C.; Leme, K.C.; da Silva, M.A.; da Rocha, C.Q.; Tangerina, M.M.P.; Vilegas, W.; Figueiredo, S.A.; da Silveria Duarte, S.M.; Rodrigues, M.R.; de Araújo Paula, F.B. Protective Effect of Flavonoids from Passiflora edulis Sims on Diabetic Complications in Rats. J. Pharm. Pharmacol. 2021, 73, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, M.; Cavé, A.; Bhaumik, P.K.; Mukherjee, B.; Mukherjee, R. The Phytochemistry of the Annonaceae. Phytochemistry 1980, 21, 2783–2813. [Google Scholar] [CrossRef]
- Moghadamtousi, S.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.; Kadir, H. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Oni, O.A.; Falayi, O.O.; Ogunmiluyi, I.O.; Ogunpolu, B.S.; Omobowale, T.O.; Oyagbemi, A.A.; Oguntibeju, O.O.; Yakubu, M.A. Annona muricata Mitigates Glycerol-Induced Nephrotoxicities in Male Albino Rats through Signaling Pathways of Angiotensin Conversion Enzyme, Kidney Injury Molecule-1, and Antioxidant Properties. Sci. Afr. 2022, 16, e01225. [Google Scholar] [CrossRef]
- Bremekamp, C.E.B. Tropicos.Org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- Conserva, L.; Ferreira, J. Borreria and Spermacoce Species (Rubiaceae): A Review of Their Ethnomedicinal Properties, Chemical Constituents, and Biological Activities. Pharmacogn. Rev. 2012, 6, 46. [Google Scholar] [CrossRef]
- Kala, S.C. Medicinal Attributes of Family Rubiaceae. Int. J. Pharm. Biol. Sci. 2015, 5, 179–181. [Google Scholar]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, Phytochemistry and Pharmacology of Uncaria (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef]
- Valente, L.M.M.; Bizarri, C.H.B.; Liechocki, S.; Barboza, R.S.; da Paixão, D.; Almeida, M.B.S.; Benevides, P.J.C.; Magalhães, A.; Siani, A.C. Kaempferitrin from Uncaria Guianensis (Rubiaceae) and Its Potential as a Chemical Marker for the Species. J. Braz. Chem. Soc. 2009, 20, 1041–1045. [Google Scholar] [CrossRef]
- Machado, D.I.; de Oliveira Silva, E.; Ventura, S.; Vattimo, M.d.F.F. The Effect of Curcumin on Renal Ischemia/Reperfusion Injury in Diabetic Rats. Nutrients 2022, 14, 2798. [Google Scholar] [CrossRef] [PubMed]
- Mostacedo, C.B.; Uslar, Y. Plantas Silvestres Con Frutos y Semillas Comestibles Del Departamento de Santa Cruz, Bolivia: Un Inventario Preliminar. Rev. Soc. Boliv. Botánica 1999, 2, 203–226. [Google Scholar]
- Sales, G.W.P.; Batista, A.H.M.; Rocha, L.Q.; Nogueira, N.A.P. Efeito Antimicrobiano e Modulador Do Óleo Essencial Extraído Da Casca de Frutos Da Hymenaea courbaril L. Rev. Ciências Farm. Básica E Apl. 2014, 35, 709–715. [Google Scholar]
- Tiago, P.V.; Larocca, D.; da Silva, I.V.; Carpejani, A.A.; Tiago, A.V.; Dardengo, J.d.F.E.; Rossi, A.A.B. Caracterização Morfoanatômica, Fitoquímica e Histoquímica de Hymenaea courbaril (Leguminosae), Ocorrente Na Amazônia meridional. Rodriguésia 2020, 71, e02182018. [Google Scholar] [CrossRef]
- Duarte, M.M.; de Paula, S.R.P.; Ferreira, F.R.d.L.; Nogueira, A.C. Morphological Characterization of Fruit, Seed and Seedling and Germination of Hymenaea courbaril L. (Fabaceae) (‘Jatobá’). J. Seed Sci. 2016, 38, 204–211. [Google Scholar] [CrossRef]
- Gorchov, D.L.; Palmeirim, J.M.; Ascorra, C.F. Dispersal of Seeds of Hymenaea courbaril (Fabaceae) in a Logged Rain Forest in the Peruvian Amazonian. Acta Amazon. 2004, 34, 251–259. [Google Scholar] [CrossRef]
- Dos Santos, A.G.; Sivieri, K.; Miglioli da Mata, B.P.; Salgaço, M.K.; Silva do Sacramento, L.V. Jatobá (Hymenaea courbaril L.). In Handbook of Phytonutrients in Indigenous Fruits and Vegetables; CABI: Wallingford, UK, 2022; pp. 266–280. [Google Scholar]
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line. Molecules 2021, 26, 6142. [Google Scholar] [CrossRef]
- Campelo, D.S.; Campelo, T.P.T.; Ferraz, A.B.F. Avaliação das Características Químicas e Biológicas da Garrafada de Carobinha; Digital Editora: Canoas, Brazil, 2021. [Google Scholar]
- Gindri-Sinhorin, V. Avaliação Antioxidante Do Extrato Da Semente de Hymenaea courbaril l. (Jatobá) Em Camundongos Tratados Com Acetaminofeno. Rev. Cuba. Plantas Med. 2020, 25, 1–13. [Google Scholar]
- de Jesus Lisboa, E.M.; Albiero, L.R.; Melchiors, N.; de Paula Borges, W.S.; da Silva Lima, V.; Rodrigues, F.D.; Sinhorin, V.D.G.; Castoldi, L. Evaluation of the Antitumor and Antioxidant Effects of Jatobá (Hymenaea courbaril) Extracts/Avaliação Do Efeito Antitumoral e Antioxidante de Extratos Do Jatobá (Hymenaea courbaril). Braz. J. Dev. 2021, 7, 116001–116018. [Google Scholar] [CrossRef]
- Micheli, M. Tropicos.org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- Portella, V.G.; Cosenza, G.P.; Diniz, L.R.L.; Pacheco, L.F.; Cassali, G.D.; Caliari, M.V.; Brandão, M.d.G.L.; Vieira, M.A.R. Nephroprotective Effect of Echinodorus macrophyllus Micheli on Gentamicin-Induced Nephrotoxicity in Rats. Nephron Extra 2012, 2, 177–183. [Google Scholar] [CrossRef]
- Dutra, R.C.; Tavares, C.Z.; Ferraz, S.O.; Sousa, O.V.; Pimenta, D.S. Investigação Das Atividades Analgésica e Antiinflamatória Do Extrato Metanólico Dos Rizomas de Echinodorus grandiflorus. Rev. Bras. Farmacogn. 2006, 16, 469–474. [Google Scholar] [CrossRef]
- Marques, A.M.; Provance, D.W.; Kaplan, M.A.C.; Figueiredo, M.R. Echinodorus grandiflorus: Ethnobotanical, Phytochemical and Pharmacological Overview of a Medicinal Plant Used in Brazil. Food Chem. Toxicol. 2017, 109, 1032–1047. [Google Scholar] [CrossRef]
- De Fafia Garcia, E.; de Oliveira, M.A.; Godin, A.M.; Ferreira, W.C.; Bastos, L.F.S.; de Matos Coelho, M.; Braga, F.C. Antiedematogenic Activity and Phytochemical Composition of Preparations from Echinodorus grandiflorus Leaves. Phytomedicine 2010, 18, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, E.L.; Watanabe, M.; da Fonseca, C.D.; Schlottfeldt, F.d.S.; Vattimo, M.d.F.F. Renoprotective Effect of the Echinodorus macrophyllus in Induced Renal Injury. Acta Paul. Enferm. 2014, 27, 12–17. [Google Scholar] [CrossRef]
- Jansen, R.K.; Tropicos.org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- Kostić, A.Ž.; Janaćković, P.; Kolašinac, S.M.; Dajić Stevanović, Z.P. Balkans’ Asteraceae Species as a Source of Biologically Active Compounds for the Pharmaceutical and Food Industry. Chem. Biodivers. 2020, 17, e2000097. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.R.; Anholeto, L.; Ferreira Rodrigues, R.; Arnosti, A.; Bechara, G.; de Carvalho Castro, K.; Camargo-Mathias, M. Cytotoxic Effects of Extract of Acmella oleracea in the Ovaries and Midgut of Rhipicephalus sanguineus Latreille, 1806 (Acari: Ixodidae) Female Ticks. J. Microsc. Ultrastruct. 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.d.S.; Vieir, M.A.; Marqu, M.O.; Vianello, F.; Lima, G.P. Influence of Organic and Mineral Soil Fertilization on Essential Oil of Spilanthes Oleracea Cv. Jambuarana. Am. J. Plant Physiol. 2012, 7, 135–142. [Google Scholar] [CrossRef]
- Da Silva Borges, L.; de Souza Vieira, M.C.; Vianello, F.; Goto, R.; Lima, G.P.P. Antioxidant Compounds of Organically and Conventionally Fertilized Jambu (Acmella oleracea). Biol. Agric. Hortic. 2016, 32, 149–158. [Google Scholar] [CrossRef]
- Ratnasooriya, W.D.; Pieris, K.P.P.; Samaratunga, U.; Jayakody, J.R.A.C. Diuretic Activity of Spilanthes acmella Flowers in Rats. J. Ethnopharmacol. 2004, 91, 317–320. [Google Scholar] [CrossRef]
- Yadav, R.; Kharya, M.D.; Yadav, N.; Savadi, R. Diuretic Activity of Spilanthes acmella Murr. Leaves Extract in Rats. Int. J. Res. Pharm. Chem. 2011, 1, 57–61. [Google Scholar]
- Gerbino, A.; Schena, G.; Milano, S.; Milella, L.; Barbosa, A.F.; Armentano, F.; Procino, G.; Svelto, M.; Carmosino, M. Spilanthol from Acmella oleracea Lowers the Intracellular Levels of CAMP Impairing NKCC2 Phosphorylation and Water Channel AQP2 Membrane Expression in Mouse Kidney. PLoS ONE 2016, 11, e0156021. [Google Scholar] [CrossRef] [PubMed]
- Zappi, D.C.; Filardi, F.L.R.; Leitman, P.; Souza, V.C.; Walter, B.M.T.; Pirani, J.R.; Morim, M.P.; Queiroz, L.P.; Cavalcanti, T.B.; Mansano, V.F.; et al. Growing Knowledge: An Overview of Seed Plant Diversity in Brazil. Rodriguésia 2015, 66, 1085–1113. [Google Scholar] [CrossRef]
- Fernandez, L.; Duque, S.; Sanchez, I.; Quiñones, D.; Rodriguez, F.; Garcia-Abujeta, J.L. Allergic Contact Dermatitis from Rosemary (Rosmarinus officinalis L.). Contact Dermat. 1997, 37, 248–249. [Google Scholar] [CrossRef]
- Emami, F.; Ali-Beig, H.; Farahbakhs, S.; Mojabi, N.; Rastegar-M, B.; Arbabian, S.; Kazemi, M.; Tekieh, E.; Golmanesh, L.; Ranjbaran, M.; et al. Hydroalcoholic Extract of Rosemary (Rosmarinus officinalis L.) and Its Constituent Carnosol Inhibit Formalin-Induced Pain and Inflammation in Mice. Pak. J. Biol. Sci. 2013, 16, 309–316. [Google Scholar] [CrossRef]
- Sotelo-Félix, J.I.; Martinez-Fong, D.; Muriel, P.; Santillán, R.L.; Castillo, D.; Yahuaca, P. Evaluation of the Effectiveness of Rosmarinus officinalis (Lamiaceae) in the Alleviation of Carbon Tetrachloride-Induced Acute Hepatotoxicity in the Rat. J. Ethnopharmacol. 2002, 81, 145–154. [Google Scholar] [CrossRef]
- Almela, L.; Sánchez-Muñoz, B.; Fernández-López, J.A.; Roca, M.J.; Rabe, V. Liquid Chromatograpic–Mass Spectrometric Analysis of Phenolics and Free Radical Scavenging Activity of Rosemary Extract from Different Raw Material. J. Chromatogr. A 2006, 1120, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zohrabi, M. The Study of 24 h Post Treatment Effects of the Aqueous Extract of Rosmarinus officinalis after Renal Ischemia/Reperfusion in Rat. J. Physiol. Pathophysiol. 2012, 3, 12–19. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; El-Sayed, R.A.; Abdel-Daim, M.M. Rosmarinus officinalis Essential Oil Modulates Renal Toxicity and Oxidative Stress Induced by Potassium Dichromate in Rats. J. Trace Elem. Med. Biol. 2021, 67, 126791. [Google Scholar] [CrossRef]


| Species | Parts Used | Isolated or Characterized Constituents | Pharmacological Activity | |
|---|---|---|---|---|
| Banisteriopsis caapi (Spruce ex Griseb.) Morton | Stem | Harmine (1), harmaline (2) [114], tetrahydroharmine (3), and harmalinic acid (4) [115] | Analgesic [22], hallucinogen [23], anesthetic [24], antidiabetic [25], anticancerogenic [18], nephroprotective, diuretic [26] | |
| Peganum harmala L. | Seeds | Harmol (5), harmalol (6), harmine (1), and harmaline (2) [116] | Antioxidant, nephroprotective, anti-inflammatory, anti-apoptotic [116] | |
| Passiflora edulis Sims | Fruit peel, leaves, flowers, seeds | Orientin (7) and isoorientin (8) [117] | Anxiolytic, sedative, neuropathic pain [118], anticonvulsant [119], cognitive function and degenerative diseases [120], antioxidant action, antitumor action, hypoglycemic action, obesity, insomnia, nephroprotector [121] | |
| Annona muricata L. | Leaves | Acetogenin (9) [122], δ-Cadinene (10), and α-Muurolene (11) [123] | Anticancerogenic, hepatoprotective, neurotoxic, antinociceptive, antiulcerative, chemopreventive, nephroprotective [124] | |
| Uncaria tomentosa (Willd.) DC. | Stem | Uncarine F (12), speciophylline (13), and mitraphylline (14) [125] | Antioxidant and immunomodulator, anti-inflammatory, analgesic, anticancer, and diuretic [126] | |
| Hymenaea courbaril L. | Stem and leaves | Fisetin (15), cyclosativene (16), caryophyllene (17), and α-himachalene (18) [127] | Antioxidant, antiulcerogenic, anti-inflammatory, antitumor, and diuretic [128] | |
| Echinodorus macrophyllus (Kunth) Micheli | Leaves | Linalool (19), α-caryophyllene (20), β-caryophyllene (21) [129], isovitexin (22), and isoorientin (8) [130] | Diuretic, anti-inflammatory, treatment of kidney and liver disorders [131] | |
| Acmella oleracea (L.) R. K. Jansen | Flowers and leaves | Spilanthol (23), spermidine (24), spermine (25), and 3-acetylaleuritolic acid (26) [132,133,134] | Aphrodisiac, treatment of male sexual dysfunctions, diuretic, and anti-inflammatory [135,136] | |
| Rosmarinus officinalis L. | Leaves | Camphene (27), limonene (28), camphor (29), borneol (30), cineol (31), and linalool (19) [137] | Analgesic, anti-inflammatory, anticarcinogenic, antirheumatic, nephroprotective, spasmolytic, antihepatotoxic, atherosclerotic [138] | |
Harmine (1) | Harmaline (2) | Tetrahydroharmine (3) | Harmalinic acid (4) | Harmol (5) |
Harmalol (6) | Orientin (7) | Isoorientin (8) | Acetogenin (9) | |
δ-Cadinene (10) | α-Muurolene (11) | Uncarine F (12) | Speciophylline (13) | Mitraphylline (14) |
Fisetin (15) | Cyclosativene (16) | Caryophyllene (17) | α-himachalene (18) | Linalool (19) |
α-caryophyllene (20) | β-caryophyllene (21) | Isovitexin (22) | Spilanthol (23) | |
Spermidine (24) | Spermine (25) | 3-acetylaleuritolic acid (26) | Camphene (27) | |
Limonene (28) | Camphor (29) | Borneol (30) | Cineol (31) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paes, A.S.; Koga, R.d.C.R.; Sales, P.F.; Santos Almeida, H.K.; Teixeira, T.A.C.C.; Carvalho, J.C.T. Phytocompounds from Amazonian Plant Species against Acute Kidney Injury: Potential Nephroprotective Effects. Molecules 2023, 28, 6411. https://doi.org/10.3390/molecules28176411
Paes AS, Koga RdCR, Sales PF, Santos Almeida HK, Teixeira TACC, Carvalho JCT. Phytocompounds from Amazonian Plant Species against Acute Kidney Injury: Potential Nephroprotective Effects. Molecules. 2023; 28(17):6411. https://doi.org/10.3390/molecules28176411
Chicago/Turabian StylePaes, Alberto Souza, Rosemary de Carvalho Rocha Koga, Priscila Faimann Sales, Hellen Karine Santos Almeida, Thiago Afonso Carvalho Celestino Teixeira, and José Carlos Tavares Carvalho. 2023. "Phytocompounds from Amazonian Plant Species against Acute Kidney Injury: Potential Nephroprotective Effects" Molecules 28, no. 17: 6411. https://doi.org/10.3390/molecules28176411