Design, Synthesis, and Antimicrobial Activity Evaluation of Ciprofloxacin—Indole Hybrids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
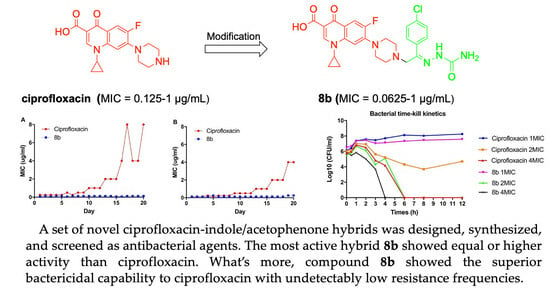
2.2. Pharmacology
2.2.1. Antibacterial Activity
2.2.2. Propensity for the Development of Bacterial Resistance
2.2.3. Evaluation of Bacterial Resistance Development
2.3. Cytotoxic Activity
2.4. Molecular Docking and Drug-like Properties Prediction
3. Materials and Methods
3.1. Chemical Part
3.1.1. Synthesis Procedure of 1-(3-Bromopropyl)-1H-indole-3-carbaldehyde (1)
3.1.2. Synthesis Procedure of Ciprofloxacin–Indole Hybrid 2
3.1.3. Synthesis Procedure of Ciprofloxacin–Indole Hybrid 3a
3.1.4. Synthesis Procedure of Ciprofloxacin–Indole Hybrid 3b
3.1.5. Synthesis Procedure of Ciprofloxacin–Indole Hybrid 3c
3.1.6. Synthesis Procedure of Ciprofloxacin–Indole Hybrid 3d
3.1.7. Synthesis Procedure of 1-(4-Bromobutyl)-1H-indole-3-carbaldehyde (4a)
3.1.8. Synthesis Procedure of 1-(5-Bromopentyl)-1H-indole-3-carbaldehyde (4b)
3.1.9. Synthesis Procedure of Ciprofloxacin–Indole Hybrid 5a
3.1.10. Synthesis Procedure of Ciprofloxacin–Indole Hybrid 5b
3.1.11. Synthesis Procedure of Ciprofloxacin–Indole Hybrid 6a
3.1.12. Synthesis Procedure of Ciprofloxacin–Indole Hybrid 6b
3.1.13. Synthesis Procedure of Ciprofloxacin–Acetophenone Hybrid 7a
3.1.14. Synthesis Procedure of Ciprofloxacin–Acetophenone Hybrid 7b
3.1.15. Synthesis Procedure of Ciprofloxacin–Acetophenone Hybrid 8a
3.1.16. Synthesis Procedure of Ciprofloxacin–Acetophenone Hybrid 8b
3.2. Pharmacological Assays
3.2.1. Antibacterial Activity Evaluation
3.2.2. Propensity Evaluation for the Development of Bacterial Resistance
3.2.3. Time-Kill Assay
3.3. Evaluation of Cytotoxicity Activity In Vitro
3.4. Molecular Docking and Drug-like Properties Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kotwani, A.; Joshi, J.; Kaloni, D. Pharmaceutical effluent: A critical link in the interconnected ecosystem promoting antimicrobial resistance. Environ. Sci. Pollut. Res. Int. 2021, 28, 32111–32124. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 28 September 2020).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Research Agenda for Antimicrobial Resistance in Human Health. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/who-global-research-agenda-for-amr-in-human-health---policy-brief.pdf?sfvrsn=f86aa073_4&download=true (accessed on 14 August 2023).
- WHO. Incentivising the Development of New Antibacterial Treatments. 2023. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-gcp-irc/incentivising-development-of-new-antibacterial-treatments-2023---progress-report.pdf?sfvrsn=72e4f738_3 (accessed on 14 August 2023).
- Chen, R.; Zhang, H.; Ma, T.; Xue, H.; Miao, Z.; Chen, L.; Shi, X. Ciprofloxacin-1,2,3-triazole-isatin hybrids tethered via amide: Design, synthesis, and in vitro anti-mycobacterial activity evaluation. Bioorg. Med. Chem. Lett. 2019, 29, 2635–2637. [Google Scholar] [CrossRef]
- Wang, R.; Yin, X.; Zhang, Y.; Yan, W. Design, synthesis and antimicrobial evaluation of propylene-tethered ciprofloxacin-isatin hybrids. Eur. J. Med. Chem. 2018, 156, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Patel, L.J. Design, Synthesis and Molecular Docking of 1-Cyclopropyl-6- Fluoro-4-Oxo-7-{4-[2-(4-Substituted-Phenyl)-2-(Substituted)-Ethyl]-1-Piperazinyl}-1,4-Dihydroquinoline-3-Carboxylic Acid as an Antimicrobial Agents. Curr. Drug Discov. Technol. 2017, 14, 255–269. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019; ISBN 9789241515528. [Google Scholar]
- ClinCalc. The Top 300 of 2019. Available online: https://clincalc.com/DrugStats/Top300Drugs.aspx (accessed on 6 July 2023).
- Zhang, G.F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.L. CIP derivatives and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Jaitak, V. Recent Development in Indole Derivatives as Anticancer Agents for Breast Cancer. Anticancer Agents Med. Chem. 2019, 19, 962–983. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Kaur, K.; Biharee, A.; Jaitak, V. Recent Development in Indole Derivatives as Anticancer Agent: A Mechanistic Approach. Anticancer Agents Med. Chem. 2021, 21, 1802–1824. [Google Scholar] [CrossRef]
- Kerzare, D.R.; Menghani, S.S.; Rarokar, N.R.; Khedekar, P.B. Development of novel indole-linked pyrazoles as anticonvulsant agents: A molecular hybridization approach. Arch. Pharm. 2021, 354, e2000100. [Google Scholar] [CrossRef]
- Archana, S.S. Synthesis and Anticonvulsant Studies of Thiazolidinone and Azetidinone Derivatives from Indole Moiety. Drug Res. 2019, 69, 445–450. [Google Scholar]
- Han, X.Y.; Zhong, Y.F.; Li, S.B.; Liang, G.C.; Zhou, G.; Wang, X.K.; Chen, B.H.; Song, Y.L. Synthesis, Characterization and Antifungal Evaluation of Novel Thiochromanone Derivatives Containing Indole Skeleton. Chem. Pharm. Bull. 2016, 64, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.L.; Chen, J.; Sun, M.; Zhang, D.B.; Gao, K. Antifungal Indole Alkaloids from Winchia calophylla. Planta. Med. 2016, 82, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.S.; Pal, M. Indole Derivatives as Anti-Tubercular Agents: An Overview on their Synthesis and Biological Activities. Curr. Med. Chem. 2021, 28, 4531–4568. [Google Scholar] [CrossRef]
- Soares, A.; Estevao, M.S.; Marques, M.M.B.; Kovalishyn, V.; Latino, D.A.R.S.; Aires-de-Sousa, J.; Ramos, J.; Viveiros, M.; Martins, F. Synthesis and Biological Evaluation of Hybrid 1,5- and 2,5-Disubstituted Indoles as Potentially New Antitubercular Agents. Med. Chem. 2017, 13, 439–447. [Google Scholar]
- Liu, Y.; Cui, Y.; Lu, L.; Gong, Y.; Han, W.; Piao, G. Natural indole-containing alkaloids and their antibacterial activities. Arch. Pharm. (Weinheim) 2020, 353, e2000120. [Google Scholar] [CrossRef]
- Song, F.; Li, Z.; Bian, Y.; Huo, X.; Fang, J.; Shao, L.; Zhou, M. Indole/isatin-containing hybrids as potential antibacterial agents. Arch. Pharm. 2020, 353, e2000143. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Hou, Y.; Shang, C.; Zhang, J.; Zhang, B. Recent advances in indole dimers and hybrids with antibacterial activity against methicillin-resistant Staphylococcus aureus. Arch. Pharm. 2021, 354, e2000266. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ye, L.; Kong, F.; Huang, G.; Xiao, J. Design, synthesis and antibacterial activity evaluation of moxifloxacin-amide-1,2,3-triazole-isatin hybrids. Bioorg. Chem. 2019, 91, 103162. [Google Scholar] [CrossRef]
- Praveen Kumar, V.; Renjitha, J.; Fathimath Salfeena, C.T.; Ashitha, K.T.; Rangappa, S.K.; Sunil, V.; Sasidhar, B.S. Antibacterial and antitubercular evaluation of dihydronaphthalenone-indole hybrid analogs. Chem. Biol. Drug. Des. 2017, 90, 703–708. [Google Scholar]
- Lin, L.; Jiang, N.; Wu, H.; Mei, Y.; Yang, J.; Tan, R. Cytotoxic and antibacterial polyketide-indole hybrids synthesized from indole-3-carbinol by Daldinia eschscholzii. Acta. Pharm. Sin. B 2019, 9, 369–380. [Google Scholar] [CrossRef]
- Oblak, M.; Grdadolnik, S.G.; Kotnik, M.; Jerala, R.; Filipič, M.; Šolmajer, T. In silico fragment- based discovery of indolin-2-one analogues as potent DNA gyrase inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5207–5210. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.J.; Deng, J.L.; Wang, Q.; Lv, Z.S. Ciprofloxacin-Isatin hybrids and their antimycobacterial activities. J. Heterocyclic. Chem. 2019, 56, 319–324. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Karaman, R.; Jubeh, B.; Breijyeh, Z. Resistance of Gram-Positive Bacteria to Current Antibacterial Agents and Overcoming Approaches. Molecules 2020, 25, 2888. [Google Scholar] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Kosowska-Shick, K.; Clark, C.; Pankuch, G.A.; McGhee, P.; Dewasse, B.; Beachel, L.; Appelbaum, P.C. Activity of telavancin against staphylococci and enterococci determined by MIC and resistance selection studies. Antimicrob Agents Chemother. 2009, 53, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.D.; Chan, P.F.; Eggleston, D.S.; Fosberry, A.; Gentry, D.R.; Gorrec, F.; Giordano, I.; Hann, M.M.; Hennessy, A.; Hibbs, M.; et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 2010, 466, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Blower, T.R.; Williamson, B.H.; Kerns, R.J.; Berger, J.M. Crystal structure and stability of gyrase-fluoroquinolone cleaved complexes from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2016, 113, 1706–1713. [Google Scholar] [CrossRef]
- Hua, Y.; Song, M.X.; Deng, X.Q. Study on Antibacterial Activity of Rosa Laevigata Michx. and Its Active Fraction. J. Liaocheng Univ. (Nat. Sci. Ed.) 2023, 36, 91–95+110. [Google Scholar]
- Song, M.; Wang, S.; Wang, Z.; Fu, Z.; Zhou, S.; Cheng, H.; Liang, Z.; Deng, X. Synthesis, antimicrobial and cytotoxic activities, and molecular docking studies of N-arylsulfonylindoles containing an aminoguanidine, a semicarbazide, and a thiosemicarbazide moiety. Eur. J. Med. Chem. 2019, 166, 108–118. [Google Scholar] [CrossRef]







| Compound | Gram-Positive Strains | Gram-Negative Strains | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 26003 a | 6538 b | 25923 c | 336931 d | 29212 e | 63501 f | 25922 g | 44568 h | 44103 i | 27853 j | 10104 k | |
| 2 | 4 | 0.5 | 0.5 | 4 | 0.5 | 0.5 | 8 | 0.5 | 0.5 | 8 | 0.5 |
| 3a | 4 | 0.25 | 0.25 | 4 | 0.5 | 1 | 8 | 0.25 | 0.25 | 4 | 0.5 |
| 3b | 4 | 4 | 4 | 16 | 32 | 2 | 16 | 8 | 4 | 8 | 8 |
| 3c | 4 | 4 | 2 | 4 | 32 | 2 | 16 | 2 | 2 | 8 | 4 |
| 3d | 4 | 2 | 1 | 4 | 8 | 1 | 16 | 1 | 1 | 8 | 2 |
| 5a | 2 | 1 | 0.5 | 8 | 1 | 1 | 4 | 1 | 1 | 4 | 2 |
| 5b | 16 | 4 | 1 | 16 | 4 | 16 | 32 | 2 | 2 | 16 | 8 |
| 6a | 8 | 0.5 | 0.5 | 8 | 1 | 4 | 16 | 0.5 | 0.5 | 8 | 2 |
| 6b | 8 | 2 | 0.5 | 16 | 8 | 8 | 16 | 1 | 4 | 16 | 16 |
| 7a | 2 | 1 | 0.5 | 2 | 1 | 1 | 2 | 2 | 2 | 8 | 4 |
| 7b | 0.5 | 0.5 | 0.125 | 1 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 2 | 1 |
| 8a | 0.5 | 0.5 | 0.25 | 2 | 0.25 | 1 | 1 | 0.25 | 0.5 | 8 | 2 |
| 8b | 0.25 | 0.25 | 0.0625 | 0.5 | 0.25 | 0.25 | 0.5 | 0.25 | 0.125 | 1 | 0.5 |
| Ciprofloxacin | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.125 | 1 | 0.5 |
| Norfloxacin | 0.5 | 0.5 | 0.5 | 16 | 1 | 2 | 2 | 0.5 | 0.5 | 2 | 4 |
| Penicillin | 0.5 | 2 | 0.5 | 2 | >32 | >32 | >32 | >32 | >32 | >32 | 32 |
| Compounds | MDR Gram-Positive Strains | MDR Gram-Negative Strains | |
|---|---|---|---|
| 43300 a | 33591 b | BAA-196 c | |
| 2 | 2 | 2 | 16 |
| 3a | 0.5 | 0.5 | 4 |
| 3b | 16 | 8 | 32 |
| 3c | 16 | 4 | 32 |
| 3d | 8 | 4 | 16 |
| 5a | 1 | 2 | 8 |
| 5b | 4 | 4 | 32 |
| 6a | 8 | 4 | 32 |
| 6b | 4 | 16 | 16 |
| 7a | 1 | 1 | 1 |
| 7b | 0.5 | 0.5 | 1 |
| 8a | 0.5 | 1 | 0.5 |
| 8b | 0.25 | 0.25 | 0.25 |
| Ciprofloxacin | 0.25 | 0.25 | 0.25 |
| Norfloxacin | 0.5 | 0.5 | 0.5 |
| Penicillin | 32 | >32 | >32 |
| Compoud | MW | CLogP | HBD | HBA | n-ROTB | Lipinski’s Violation |
|---|---|---|---|---|---|---|
| Rule | ≤500 | ≤5 | ≤5 | <10 | ≤10 | ≤1 |
| 2 | 516.57 | 1.80 | 1 | 8 | 8 | 1 |
| 3a | 573.63 | 1.41 | 4 | 11 | 9 | 2 |
| 3b | 589.70 | 1.95 | 4 | 10 | 10 | 1 |
| 3c | 634.71 | 3.17 | 2 | 10 | 10 | 1 |
| 3d | 545.62 | 1.80 | 1 | 9 | 9 | 1 |
| 5a | 530.60 | 2.07 | 1 | 8 | 9 | 1 |
| 5b | 544.63 | 2.67 | 1 | 8 | 10 | 1 |
| 6a | 587.66 | 1.68 | 4 | 11 | 10 | 2 |
| 6b | 601.18 | 2.19 | 4 | 11 | 11 | 3 |
| 7a | 479.51 | 1.10 | 1 | 8 | 7 | 0 |
| 7b | 483.93 | 1.72 | 1 | 7 | 6 | 0 |
| 8a | 536.56 | 0.52 | 4 | 11 | 8 | 2 |
| 8b | 540.98 | 1.14 | 4 | 10 | 7 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Hua, Y.; Liu, Y.; Xiao, X.; Yu, H.; Deng, X. Design, Synthesis, and Antimicrobial Activity Evaluation of Ciprofloxacin—Indole Hybrids. Molecules 2023, 28, 6325. https://doi.org/10.3390/molecules28176325
Song M, Hua Y, Liu Y, Xiao X, Yu H, Deng X. Design, Synthesis, and Antimicrobial Activity Evaluation of Ciprofloxacin—Indole Hybrids. Molecules. 2023; 28(17):6325. https://doi.org/10.3390/molecules28176325
Chicago/Turabian StyleSong, Mingxia, Yi Hua, Yuxin Liu, Xunli Xiao, Haihong Yu, and Xianqing Deng. 2023. "Design, Synthesis, and Antimicrobial Activity Evaluation of Ciprofloxacin—Indole Hybrids" Molecules 28, no. 17: 6325. https://doi.org/10.3390/molecules28176325