Utilization of Fe-Ethylenediamine-N,N′-Disuccinic Acid Complex for Electrochemical Co-Catalytic Activation of Peroxymonosulfate under Neutral Initial pH Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of ACA Degradation in Different Systems
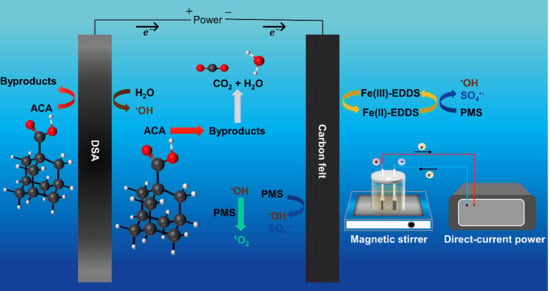
2.2. Oxidation Mechanisms of the EC/PMS/Fe(III)-EDDS System
2.3. ACA Degradation Pathways and Toxicity Assessment of Degradation Byproducts
2.4. Degradation of Fluka Commercial NA Mixture
3. Materials and Methods
3.1. Chemicals
3.2. Degradation Experiment
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zargar, M.; Ahmadinia, E.; Asli, H.; Karim, M.R. Investigation of the possibility of using waste cooking oil as a rejuvenating agent for aged bitumen. J. Hazard. Mater. 2012, 233–234, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.W. Process water treatment in Canada’s oil sands industry: I. Target pollutants and treatment objectives. J. Environ. Eng. Sci. 2008, 7, 123–138. [Google Scholar] [CrossRef]
- Hagen, M.O.; Garcia-Garcia, E.; Oladiran, A.; Karpman, M.; Mitchell, S.; El-Din, M.G.; Martin, J.W.; Belosevic, M. The acute and sub-chronic exposures of goldfish to naphthenic acids induce different host defense responses. Aquat. Toxicol. 2012, 109, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Debenest, T.; Turcotte, P.; Gagné, F.; Gagnon, C.; Blaise, C. Ecotoxicological impacts of effluents generated by oil sands bitumen extraction and oil sands lixiviation on Pseudokirchneriella subcapitata. Aquat. Toxicol. 2012, 112–113, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Z.; Fang, C.; Liu, W.; Lou, X.; Liu, J. Importance of reagent addition order in contaminant degradation in an Fe(ii)/PMS system. RSC Adv. 2016, 6, 70271–70276. [Google Scholar] [CrossRef]
- Pérez-Estrada, L.A.; Han, X.; Drzewicz, P.; Gamal El-Din, M.; Fedorak, P.M.; Martin, J.W. Structure–Reactivity of Naphthenic Acids in the Ozonation Process. Environ. Sci. Technol. 2011, 45, 7431–7437. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. Oxidative degradation of Orange G by peroxomonosulfate in presence of biosynthesized copper nanoparticles—A kinetic study. Environ. Technol. Innov. 2018, 10, 281–289. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Tang, C.; Chen, J.; Liu, G. Heat/PMS Degradation of Atrazine: Theory and Kinetic Studies. Processes 2022, 10, 941. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef]
- Song, J.; How, Z.T.; Huang, Z.; El-Din, M.G. Biochar/iron oxide composite as an efficient peroxymonosulfate catalyst for the degradation of model naphthenic acids compounds. Chem. Eng. J. 2022, 429, 132220. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Lu, Y.-S.; Luo, Z.-N.; Sun, W.-J.; Xu, B.; Hu, C.-Y.; Tang, Y.-L.; Dong, Z.-Y.; Ren, X.-M. Micropollutant removal and disinfection byproduct control by sequential peroxymonosulfate-UV treatment in water: A case study with sulfamethoxazole. J. Environ. Sci. 2022, 117, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Long, X.; Huang, R.; Zhang, I.Y.; Yao, G.; Lai, B.; Xiong, Z. Highly efficient electro-cocatalytic Fenton-like reactions for the degradation of recalcitrant naphthenic acids: Exploring reaction mechanisms and environmental implications. Chem. Eng. J. 2022, 450, 138331. [Google Scholar] [CrossRef]
- Huang, W.; Brigante, M.; Wu, F.; Mousty, C.; Hanna, K.; Mailhot, G. Assessment of the Fe(III)-EDDS complex in Fenton-like processes: From the radical formation to the degradation of bisphenol A. Environ. Sci. Technol. 2013, 47, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Yip, T.C.; Tsang, D.C.; Ng, K.T.; Lo, I.M. Kinetic Interactions of EDDS with Soils. 1. Metal Resorption and Competition under EDDS Deficiency. Environ. Sci. Technol. 2009, 43, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Williams, D.R. Chemical speciation used to assess [S,S0]-ethylenediaminedisuccinic acid (EDDS) as a readily-biodegradable replacement for EDTA in radiochemical decontamination formulations. Appl. Radiat. Isot. 2001, 54, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Klamerth, N.; Messele, S.A.; Chelme-Ayala, P.; El-Din, M.G. Kinetics study on the degradation of a model naphthenic acid by ethylenediamine-N,N’-disuccinic acid-modified Fenton process. J. Hazard. Mater. 2016, 318, 371–378. [Google Scholar] [CrossRef]
- Ye, Z.; Brillas, E.; Centellas, F.; Cabot, P.L.; Sirés, I. Electro-Fenton process at mild pH using Fe(III)-EDDS as soluble catalyst and carbon felt as cathode. Appl. Catal. B Environ. 2019, 257, 117907. [Google Scholar] [CrossRef]
- Messele, S.A.; Chelme-Ayala, P.; El-Din, M.G. Catalytic ozonation of naphthenic acids in the presence of carbon-based metal-free catalysts: Performance and kinetic study. Catal. Today 2021, 361, 102–108. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Misiti, T.M.; Pavlostathis, S.G. Removal and toxicity reduction of naphthenic acids by ozonation and combined ozonation-aerobic biodegradation. Bioresour. Technol. 2015, 179, 339–347. [Google Scholar] [CrossRef]
- Qin, R.; How, Z.T.; El-Din, M.G. Photodegradation of naphthenic acids induced by natural photosensitizer in oil sands process water. Water Res. 2019, 164, 114913. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kang, J.; Li, S.; Zhang, J.; Tang, Y.; Liu, S.; Liu, J.; Tang, P. Electro-assisted heterogeneous activation of peroxymonosulfate by g-C3N4 under visible light irradiation for tetracycline degradation and its mechanism. Chem. Eng. J. 2022, 436, 135278. [Google Scholar] [CrossRef]
- Thamilselvan, A.; Dang, V.D.; Doong, R.-A. Ni-Co bimetallic decorated dodecahedral ZIF as an efficient catalyst for photoelectrochemical degradation of sulfamethoxazole coupled with hydrogen production. Sci. Total Environ. 2023, 873, 162208. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Xiong, Z.; Huang, R.; Yu, Y.; Zhou, P.; Zhang, H.; Yao, G.; Lai, B. Sustainable Fe(III)/Fe(II) cycles triggered by co-catalyst of weak electrical current in Fe(III)/peroxymonosulfate system: Collaboration of radical and non-radical mechanisms. Appl. Catal. B Environ. 2022, 317, 121716. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, J.; Zhang, G.; Li, W.; Liu, Y.; Cheng, X.; Huo, X.; Liu, Y.; Zhang, Y. Degradation of dimethyl phthalate by activating peroxymonosulfate using nanoscale zero valent tungsten: Mechanism and degradation pathway. Chem. Eng. J. 2019, 359, 138–148. [Google Scholar] [CrossRef]
- Han, X.; Zhang, W.; Li, S.; Cheng, C.; Yu, Q.; Jia, Q.; Zhou, L.; Xiu, G. Mn-MOF derived manganese sulfide as peroxymonosulfate activator for levofloxacin degradation: An electron-transfer dominated and radical/nonradical coupling process. J. Environ. Sci. 2023, 130, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xiong, Z.; Liu, R.; He, C.; Liu, Y.; Pan, Z.; Yao, G.; Lai, B. Pivotal roles of N-doped carbon shell and hollow structure in nanoreactor with spatial confined Co species in peroxymonosulfate activation: Obstructing metal leaching and enhancing catalytic stability. J. Hazard. Mater. 2022, 427, 128204. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, H.; Xiong, Z.; Liu, Y.; Yao, G.; Lai, B. Heterogeneous activation of peroxymonosulfate by CoMgFe-LDO for degradation of carbamazepine: Efficiency, mechanism and degradation pathways. Chem. Eng. J. 2019, 391, 123604. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Zhou, H.; Lai, L.; Wan, Y.; He, Y.; Yao, G.; Lai, B. Molybdenum disulfide (MoS2): A versatile activator of both peroxymonosulfate and persulfate for the degradation of carbamazepine. Chem. Eng. J. 2020, 384, 123264. [Google Scholar] [CrossRef]
- Huang, G.-X.; Wang, C.-Y.; Yang, C.-W.; Guo, P.-C.; Yu, H.-Q. Degradation of Bisphenol A by Peroxymonosulfate Catalytically Activated with Mn(1.8)Fe(1.2)O(4) Nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wu, Z.; Zhou, H.; Li, J.; Zhou, C.; Xiong, Z.; Pan, Z.; Yao, G.; Lai, B. Recent advances in single-atom catalysts for advanced oxidation processes in water purification. J. Hazard. Mater. 2021, 412, 125253. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Chaplin, B.P. Mechanistic Study of the Validity of Using Hydroxyl Radical Probes to Characterize Electrochemical Advanced Oxidation Processes. Environ. Sci. Technol. 2017, 51, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhang, H.; Wei, X.; Zhang, Y.; Lai, B. Efficient degradation of atrazine by Co-NZ catalyst prepared by electroless plating in the presence of peroxymonosulfate: Characterization, performance and mechanistic consideration. Chem. Eng. J. 2019, 359, 1316–1326. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Hussain, I.; Huang, S.; Huang, W. Insight into reactive oxygen species in persulfate activation with copper oxide: Activated persulfate and trace radicals. Chem. Eng. J. 2017, 313, 1023–1032. [Google Scholar] [CrossRef]
- Hayat, W.; Zhang, Y.; Huang, S.; Hussain, I.; Huang, R. Insight into the degradation of methomyl in water by peroxymonosulfate. J. Environ. Chem. Eng. 2021, 9, 105358. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Wang, Y.; Zhang, I.Y.; Huang, R. Efficient removal of recalcitrant naphthenic acids with electro-cocatalytic activation of peroxymonosulfate by Fe(III)-nitrilotriacetic acid complex under neutral initial pH condition. J. Hazard. Mater. 2023, 455, 131524. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Cheng, Z.; Yuan, T.; Shen, Z. Catalytic oxidation of various aromatic compounds in supercritical water: Experimental and DFT study. J. Taiwan Inst. Chem. Eng. 2019, 100, 47–55. [Google Scholar] [CrossRef]
- Díaz, M.G.; Andrada, M.F.; Vega-Hissi, E.G.; Martinez, J.C.G. Density functional theory study of the oxidation reaction in the gas and aqueous phase of allyl methyl disulfide with hydroxyl radical. Struct. Chem. 2018, 30, 237–245. [Google Scholar] [CrossRef]
- Jiang, F.; Wu, W.; Zhu, Z.; Zhu, S.; Wang, H.; Zhang, L.; Fan, Z.; Chen, Y. Structure identification and toxicity evaluation of one newly-discovered dechlorinated photoproducts of chlorpyrifos. Chemosphere 2022, 301, 134822. [Google Scholar] [CrossRef]
- Li, W.; Guo, H.; Wang, C.; Zhang, Y.; Cheng, X.; Wang, J.; Yang, B.; Du, E. ROS reevaluation for degradation of 4-chloro-3,5-dimethylphenol (PCMX) by UV and UV/persulfate processes in the water: Kinetics, mechanism, DFT studies and toxicity evolution. Chem. Eng. J. 2020, 390, 124610. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Gao, Y.; Liu, X.; Wei, Y.; Liang, Z. Enhanced atrazine degradation in the Fe(III)/peroxymonosulfate system via accelerating Fe(II) regeneration by benzoquinone. Chem. Eng. J. 2022, 427, 131995. [Google Scholar] [CrossRef]
- Huang, R.; Chen, Y.; El-Din, M.G. Silver-Ion Solid Phase Extraction Separation of Classical, Aromatic, Oxidized, and Heteroatomic Naphthenic Acids from Oil Sands Process-Affected Water. Environ. Sci. Technol. 2016, 50, 6433–6441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Klamerth, N.; Chelme-Ayala, P.; El-Din, M.G. Comparison of Nitrilotriacetic Acid and [S,S]-Ethylenediamine-N,N′-disuccinic Acid in UV-Fenton for the Treatment of Oil Sands Process-Affected Water at Natural pH. Environ. Sci. Technol. 2016, 50, 10535–10544. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Meng, L.; How, Z.T.; Chelme-Ayala, P.; Yang, L.; Benally, C.; El-Din, M.G. Treatment of oil sands process water by the ferric citrate under visible light irradiation. Chem. Eng. J. 2022, 429, 132419. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, P.; Zhou, H.; Liu, W.; Zhang, H.; Zhou, C.; Lai, L.; Ao, Z.; Su, S.; Lai, B. Insights into the Electron-Transfer Mechanism of Permanganate Activation by Graphite for Enhanced Oxidation of Sulfamethoxazole. Environ. Sci. Technol. 2021, 55, 9189–9198. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Olah, J.; Van Alsenoy, C.; Sannigrahi, A.B. Condensed fukui functions derived from stockholder charges: Assessment of their performance as local reactivity descriptors. J. Phys. Chem. A 2002, 106, 3885–3890. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19. [Google Scholar] [CrossRef]
- Abdalrhman, A.S.; Wang, C.; How, Z.T.; El-Din, M.G. Degradation of cyclohexanecarboxylic acid as a model naphthenic acid by the UV/chlorine process: Kinetics and by-products identification. J. Hazard. Mater. 2021, 402, 123476. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Chen, Y.; Wang, Y.; Zhang, I.Y.; Huang, R. Utilization of Fe-Ethylenediamine-N,N′-Disuccinic Acid Complex for Electrochemical Co-Catalytic Activation of Peroxymonosulfate under Neutral Initial pH Conditions. Molecules 2023, 28, 6290. https://doi.org/10.3390/molecules28176290
Zhang B, Chen Y, Wang Y, Zhang IY, Huang R. Utilization of Fe-Ethylenediamine-N,N′-Disuccinic Acid Complex for Electrochemical Co-Catalytic Activation of Peroxymonosulfate under Neutral Initial pH Conditions. Molecules. 2023; 28(17):6290. https://doi.org/10.3390/molecules28176290
Chicago/Turabian StyleZhang, Bolin, Yu Chen, Yongjian Wang, Igor Ying Zhang, and Rongfu Huang. 2023. "Utilization of Fe-Ethylenediamine-N,N′-Disuccinic Acid Complex for Electrochemical Co-Catalytic Activation of Peroxymonosulfate under Neutral Initial pH Conditions" Molecules 28, no. 17: 6290. https://doi.org/10.3390/molecules28176290