Large-Scale Synthesis of Tunable Fluorescent Carbon Dots Powder for Light-Emitting Diodes and Fingerprint Identification
Abstract
:1. Introduction
2. Results and Discussion
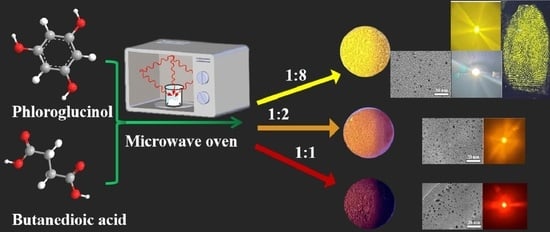
2.1. Synthesis of Y-CDs, O-CDs and R-CDs
2.2. Characterization of Y-CDs, O-CDs and R-CDs
2.3. The Biocompatibility of Y-CDs, O-CDs, R-CDs
2.4. Application of Y-CDs, O-CDs, and R-CDs in Multi-Color/White LEDs
2.5. Fingerprint Identification Application
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Preparation of Y-CDs Fluorescent Powder
3.4. Preparation of O-CDs Fluorescent Powder
3.5. Preparation of R-CDs Fluorescent Powder
3.6. MTT Assay
3.7. The Fabrication of LEDs Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, S.F.; Hou, Z.W.; Lin, L.H.; Li, F.; Zhao, Y.; Li, X.Z.; Zhang, H.; Fang, H.H.; Li, Z.C.; Sun, H.B. 3D nanoprinting of semiconductor quantum dots by photoexcitation-induced chemical bonding. Science 2022, 377, 1112–1116. [Google Scholar] [CrossRef]
- Pan, P.Y.; Liu, L.L.; Zhang, L.D.; Wei, X.; Tian, Y.P.; Kang, X.; Zhang, Q.; Zhu, M.Z. Control the single-, two-, and three-photon excited fluorescence of atomically precise metal nanoclusters. Angew. Chem. Int. Ed. 2022, 61, e202213016. [Google Scholar]
- Marjia, M.; Artiom, S.; Alma, R.G.; Antonio, B.; Fiorenzo, V. The coming of age of neodymium: Redefining its role in rare earth doped nanoparticles. Chem. Rev. 2023, 123, 515–554. [Google Scholar]
- Wu, X.G.; Ji, H.L.; Yan, X.L.; Zhong, H.Z. Industry outlook of perovskite quantum dots for display applications. Nat. Nanotechnol. 2022, 17, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Najar, A.; Wang, K.; Du, M.Y.; Liu, S.Z. Perovskite quantum dots in solar cells. Adv. Sci. 2022, 9, 2104577. [Google Scholar] [CrossRef]
- Tian, J.Y.; Tan, Q.Y.; Wang, Y.T.; Yang, Y.H.; Yuan, G.H.; Adamo, G.; Soci, C. Perovskite quantum dot one-dimensional topological laser. Nat. Common. 2023, 14, 1433. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.C.; Li, Y.H.; Yang, Y.G.; Chan, P.F.; Li, S.; Qin, Z.T.; Guo, X.Y.; Shu, L.; Fan, Z.Y.; Su, C.J.; et al. Regulating the crystallization kinetics and lattice strain of lead-free perovskites with perovskite quantum dots. ACS Energy Lett. 2022, 7, 3251–3259. [Google Scholar] [CrossRef]
- Paithankar, J.G.; Kushalan, S.; Hegde, S.; Kini, S.; Sharma, A. Systematic toxicity assessment of CdTe quantum dots in Drosophila melanogaster. Chemosphere 2022, 295, 133836. [Google Scholar] [CrossRef]
- Yao, X.X.; Lewis, R.E.; Haynes, C. Synthesis processes, photoluminescence mechanism, and the toxicity of amorphous or polymeric carbon dots. Acc. Chem. Res. 2022, 55, 3312–3321. [Google Scholar] [CrossRef] [PubMed]
- Sobhanan, J.; Rival, J.V.; Anas, A.; Shibu, E.S.; Takano, Y.; Biju, V. Luminescent quantum dots: Synthesis, optical properties, bioimaging and toxicity. Adv. Drug Deliver. Rev. 2023, 197, 114830. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Xu, M.; Hu, X.; Li, S.; Zhang, M.; Zhang, Q.; Wang, Q.; Lu, S.; Tian, Y.; et al. UV-vis-NIR full-range responsive carbon dots with large multiphoton absorption cross sections and deep-red fluorescence at nucleoli and in vivo. Small 2020, 16, 2000680. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zeng, Q.S.; Tao, S.Y.; Xia, C.L.; Liu, C.M.; Liu, C.M.; Liu, P.Y.; Yang, B. Carbon dots based photoinduced reactions: Advances and perspective. Adv. Sci. 2023, 10, 2207621. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Gong, X. The emerging development of multicolor carbon dots. Small 2022, 18, 2205099. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Wu, T.; Duan, L.Z.; Hu, G.Z.; Shi, J.H.; Nie, Y.M.; Zou, Y.M. Synthesizing carbon dots with functional preservation strategy as a facile ratiometric fluorescent sensing platform for monitoring hypochlorite in living cells and zebrafish. Sensor. Actuat. B-Chem. 2022, 365, 131946. [Google Scholar] [CrossRef]
- Lin, J.; Huang, X.; Kou, E.; Cai, W.; Zhang, H.; Zhang, X.; Liu, Y.; Li, W.; Zheng, Y.; Lei, B. Carbon dot based sensing platform for real-time imaging Cu2+ distribution in plants and environment. Biosensor. Bioelectron. 2023, 219, 114848. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Li, X.C.; Zhao, S.J.; Wang, B.H.; Song, X.Z.; Xiao, J.F.; Lan, M.H. Synthesis strategies, luminescence mechanisms, and biomedical applications of near-infrared fluorescent carbon dots. Coordin. Chem. Rev. 2022, 470, 214703. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Xu, L.L.; Wang, X.; Wang, Z.Z.; Liu, Y.; Wang, Y.; Wang, Q.L.; Wang, Z.T.; Huang, H.; Liu, Y.; et al. A comprehensive understanding on the roles of carbon dots in metallated graphyne based catalyst for photoinduced H2O2 production. Nano Today 2022, 43, 101428. [Google Scholar] [CrossRef]
- Wang, B.Y.; Yu, J.K.; Sui, L.Z.; Zhu, S.J.; Tang, Z.Y.; Yang, B.; Lu, S.Y. Rational design of multi-color-emissive carbon dots in a single reaction system by hydrothermal. Adv. Sci. 2021, 8, 2001453. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.T.; Yin, L.Q.; Liu, Y.J.; Guo, H.Z.; Lai, J.W.; Han, Y.; Li, G.; Zhang, J.H.; Vajtai, R.; et al. Full-color fluorescent carbon quantum dots. Sci. Adv. 2020, 6, eabb6772. [Google Scholar] [CrossRef]
- Han, B.Y.; Jiang, J.M.; Yan, Q.F.; Xin, Z.; Yan, Q. One-step straightfoward solid synthesis of high yield white fluorescent carbon dots for white light emitting diodes. Chin. Chem. Lett. 2021, 32, 591–593. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, B.; Lin, Y.; Wang, W.; Shiral Fernando, K.A.; Pathak, P.; Jaouad Meziani, M.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, X.; Wang, Z.; Wang, X.; Guo, G.; Pu, Q. Anomalous enhancement of fluorescence of carbon dots through lanthanum doping and potential application in intracellular imaging of ferric ion. Nano Res. 2018, 11, 1369–1378. [Google Scholar] [CrossRef]
- Egorova, M.; Tomskaya, A.; Smagulova, S.A. Optical properties of carbon dots synthesized by the hydrothermal method. Materials 2023, 16, 4018. [Google Scholar] [CrossRef]
- Xu, B.; Li, J.; Zhang, J.; Xing, H.Y.; Fang, X.Q.; Shen, J.; Zhou, H.; Jiang, T.L.; Gao, Z.H.; Meng, X.G.; et al. Solid-state fluorescent carbon dots with unprecedented efficiency from visible to near-infrared region. Adv. Sci. 2022, 10, 2205788. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, H.Y.; Jia, H.R.; Zheng, M.Y.; Xu, K.X.; Yu, R.N.; Qu, S.N.; Tan, Z.A. Triphenylamine-derived solid-state emissive carbon dots for multicolor high-efficiency electroluminescent light-emitting diodes. Angew. Chem. Int. Ed. 2023, 62, e202301651. [Google Scholar] [CrossRef]
- Dong, X.Y.; Niu, X.Q.; Zhang, Z.Y.; Wei, J.S.; Xiong, H.M. Red fluorescent carbon dot powder for accurate latent fingerprint identification using an artificial intelligence program. ACS Appl. Mater. Interfaces 2020, 12, 29549–29555. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Huang, Z.H.; Ding, L.F.; Yang, F.Y.; Peng, D. Carbon dot powders with cross-linking-based long-wavelength emission for multicolor imaging of latent fingerprints. ACS Appl. Nano Mater. 2022, 5, 2214–2221. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Wang, W.; Kong, W.G.; Chao, X.K.; Bi, Y.Y.; Li, Z.H. Metal formate framework-assisted solid fluorescent material based on carbonized nanoparticles for the detection of latent fingerprints. Anal. Chim. Acta 2022, 1209, 339864. [Google Scholar] [CrossRef]
- Guo, J.Z.; Lu, Y.S.; Xie, A.Q.; Li, G.; Liang, Z.B.; Wang, C.F.; Yang, X.N.; Chen, S. Yellow-emissive carbon dots with high solid-state photoluminescence. Adv. Funct. Mater. 2022, 32, 2110393. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, L.; Yang, L.; Wu, X.R.; Zhang, C.; Wei, K.; He, L.F.; Han, X.Y.; Qiao, H.B.; Asiri, A.M.; et al. Orange-red, green, and blue fluorescence carbon dots for white light emitting diodes. J. Mater. Sci. Technol. 2020, 50, 184–191. [Google Scholar] [CrossRef]
- Ai, L.; Song, Z.Q.; Nie, M.J.; Yu, J.K.; Liu, F.K.; Song, H.Q.; Zhang, B.; Waterhouse, G.I.N.; Lu, S.Y. Solid-state fluorescence from carbon dots widely tunable from blue to deep red through surface ligand modulation. Angew. Chem. Int. Ed. 2022, 62, e202217822. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.Z.; Wang, Y.; Zhang, S.; Ding, H.; Zhou, Z.Y.; Wei, J.S.; Li, X.H.; Xiong, H.M. Rational synthesis of silane-functionalized carbon dots with high-efficiency full-color solid-state fluorescence for light emitting diodes. Carbon 2023, 203, 1–10. [Google Scholar] [CrossRef]
- Guo, G.Q.; Li, T.T.; Wang, Y.R.; Hu, H.W.; Xing, H.M.; Tang, S.Y.; Gao, S.N.; Leng, X. Aggregation-induced bimodal excitation of nitrogen-doped carbon dots for ratiometric sensing of new coccine and solid-state multicolor lighting. J. Colloid. Interf. Sci. 2023, 645, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zheng, J.X.; Yang, Y.Z.; Liu, X.G.; Qiu, J.S.; Tian, Y. Tunable full-color solid-state fluorescent carbon dots for light emitting diodes. Carbon 2022, 190, 22–31. [Google Scholar]
- Niu, X.Q.; Song, T.B.; Xiong, H.M. Large scale synthesis of red emissive carbon dots powder by solid state reaction for fingerprint identification. Chin. Chem. Lett. 2021, 32, 1953–1956. [Google Scholar] [CrossRef]
- Niu, X.Q.; Zheng, W.J.; Song, T.B.; Huang, Z.H.; Yang, C.L.; Zhang, L.M.; Li, W.; Xiong, H.M. Pyrolysis of single carbon sources in SBA-15: A recyclable solid phase synthesis to obtain uniform carbon dots with tunable luminescence. Chin. Chem. Lett. 2023, 34, 107560. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Lu, X.F.; Liu, M.L.; Wang, W.J. Blue, yellow, and red carbon dots from aromatic precursors for light-emitting diodes. Molecules 2023, 28, 2957. [Google Scholar]
- Wang, X.; Zhao, L.; Hu, J.S.; Wei, H.; Liu, X.Y.; Li, E.S.; Yang, S.H. Rational design of novel carbon-oxygen quantum dots for ratiometrically mapping pH and reactive oxygen species scavenging. Carbon 2022, 190, 115–124. [Google Scholar]
- Feng, X.T.; Zhao, Y.Q.; Yan, L.P.; Zhang, Y.; He, Y.H.; Yang, Y.Z.; Liu, X.G. Low-temperature hydrothermal synthesis of green luminescent carbon quantum dots (CQD), and optical properties of blends of the CQD with poly(3-hexylthiophene). J. Electron. Mater. 2015, 44, 3436–3443. [Google Scholar]
- Peng, M.; Sun, S.B.; Xu, B.; Deng, Z.T. Polymer-encapsulated halide perovskite color converters to overcome blue overshoot and cyan gap of white light-emitting diodes. Adv. Funct. Mater. 2023, 33, 2300583. [Google Scholar] [CrossRef]
- Yi, H.; Liu, J.; Yao, J.; Wang, R.X.; Shi, W.Y.; Lu, C. Photoluminescence mechanism of carbon dots: Triggering multiple color emissions through controlling the degree of protonation. Molecules 2022, 27, 6517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Wang, K.P.; Dai, F.X.; Zhang, K.; Tang, H.F.; Wang, L.; Xing, J. A warm-white light-emitting diode based on single-component emitter aromatic carbon nitride. Nat. Commun. 2022, 13, 6495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Yang, Y.; Chen, L.X.; Jiang, H.H.; Hum, Y.H.; Lan, Z.J.; Miao, Y.Q.; Wang, Y.D.; Lei, Y.L.; Zhu, F.R. Single-component electroluminescent white light-emitting diodes based on zinc oxide quantum dots with high color rendition and tunable correlated color temperature. J. Mater. Chem. C 2023, 11, 5402–5410. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhang, D.; Wang, X.; Li, Y.; Li, Z.; Wei, H.; Yao, B.; Ding, G.; Wang, Z. Large-Scale Synthesis of Tunable Fluorescent Carbon Dots Powder for Light-Emitting Diodes and Fingerprint Identification. Molecules 2023, 28, 5917. https://doi.org/10.3390/molecules28155917
Zhao L, Zhang D, Wang X, Li Y, Li Z, Wei H, Yao B, Ding G, Wang Z. Large-Scale Synthesis of Tunable Fluorescent Carbon Dots Powder for Light-Emitting Diodes and Fingerprint Identification. Molecules. 2023; 28(15):5917. https://doi.org/10.3390/molecules28155917
Chicago/Turabian StyleZhao, Lei, Dong Zhang, Xin Wang, Yang Li, Zihan Li, Hua Wei, Boxuan Yao, Gongtao Ding, and Zifan Wang. 2023. "Large-Scale Synthesis of Tunable Fluorescent Carbon Dots Powder for Light-Emitting Diodes and Fingerprint Identification" Molecules 28, no. 15: 5917. https://doi.org/10.3390/molecules28155917