The Role of Cerium Valence in the Conversion Temperature of H2Ti3O7 Nanoribbons to TiO2-B and Anatase Nanoribbons, and Further to Rutile
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of H2Ti3O7 Nanoribbons Impregnated/Intercalated with Cerium
2.2. Conversion of H2Ti3O7 Nanoribbons to TiO2 by Thermal Treatment in Air
2.2.1. Cerium Content
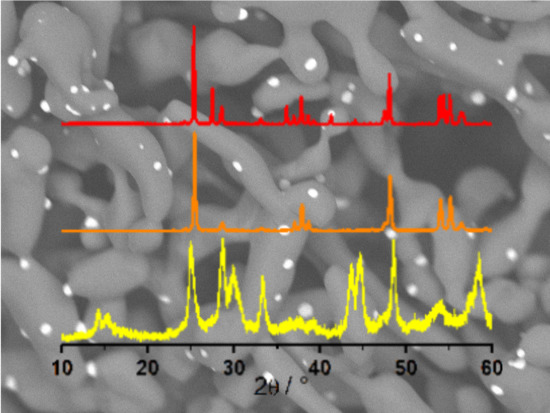
2.2.2. Structural Determination of TiO2 Polymorphs
2.2.3. Changes in the Nanoribbon Morphology and Formation of CeO2 Nanoparticles
2.2.4. Determination of Cerium Oxidation State and the Ratio between Ce4+/Ce3+
2.2.5. Optical Band Gap Features
3. Materials and Methods
3.1. Materials Preparation
3.2. Materials Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Parrino, F.; Francesca, R.P.; Giovanni, C.-R.; Vittorio, L.; Leonardo, P. 2—Properties of Titanium Dioxide. In Titanium Dioxide (TiO₂) and Its Applications; Francesco, P., Leonardo, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 13–66. [Google Scholar]
- Rahimi, N.; Randolph, A.P.; Evan, M.G. Review of Functional Titanium Oxides. I: TiO2 and Its Modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Wu, X. Applications of Titanium Dioxide Materials. In Titanium Dioxide; Hafiz Muhammad, A., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 9. [Google Scholar]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, L.Y.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and titania nanostructured materials for environmental and energy applications: A review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11, 1719. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Huang, J.; Li, Q.; Yang, J. Band Structure Tuning of TiO2 for Enhanced Photoelectrochemical Water Splitting. J. Phys. Chem. C 2014, 118, 7451–7457. [Google Scholar] [CrossRef]
- Manchwari, S.; Khatter, J.; Chauhan, R.P. Modifications in structural, morphological and optical properties of TiO2 nanoparticles: Effect of pH. Chem. Pap. 2022, 76, 7545–7551. [Google Scholar] [CrossRef]
- Jian, Z.; Pu, Y.; Fang, J.; Ye, Z. Microemulsion Synthesis of Nanosized TiO2 Particles Doping with Rare-Earth and their Photocatalytic Activity. Photochem. Photobiol. 2010, 86, 1016–1021. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, modification and application of titanium dioxide nanoparticles: A review. Nanoscale 2022, 14, 6709–6734. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.u.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, J.; Sang, Y.; Cabot, A.; Liu, H. Structure, Synthesis, and Applications of TiO2 Nanobelts. Adv. Mater. 2015, 27, 2557–2582. [Google Scholar] [CrossRef] [PubMed]
- Rutar, M.; Rozman, N.; Pregelj, M.; Bittencourt, C.; Cerc Korošec, R.; Sever Škapin, A.; Mrzel, A.; Škapin, S.D.; Umek, P. Transformation of hydrogen titanate nanoribbons to TiO2 nanoribbons and the influence of the transformation strategies on the photocatalytic performance. Beilstein J. Nanotechnol. 2015, 6, 831–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Fang, J.; Bao, H.; He, B.; Wang, F.; Si, D.; Jiang, Z.; Pan, Z.; Wei, S.; Huang, W. Interfacial and Surface Structures of CeO2–TiO2 Mixed Oxides. J. Phys. Chem. C 2007, 111, 19078–19085. [Google Scholar] [CrossRef]
- Artiglia, L.; Agnoli, S. Cerium Oxide Nanostructures on Titania: Effect of the Structure and Stoichiometry on the Reactivity Toward Ethanol Oxidation. J. Phys. Chem. C 2018, 122, 20809–20816. [Google Scholar] [CrossRef]
- Gionco, C.; Paganini, M.C.; Agnoli, S.; Reeder, A.E.; Giamello, E. Structural and spectroscopic characterization of CeO2–TiO2 mixed oxides. J. Mater. Chem. A 2013, 1, 10918–10926. [Google Scholar] [CrossRef]
- Kočí, K.; Matějová, L.; Ambrožová, N.; Šihor, M.; Troppová, I.; Čapek, L.; Kotarba, A.; Kustrowski, P.; Hospodková, A.; Obalová, L. Optimization of cerium doping of TiO2 for photocatalytic reduction of CO2 and photocatalytic decomposition of N2O. J. Sol-Gel Sci. Technol. 2016, 78, 550–558. [Google Scholar] [CrossRef]
- Veziroglu, S.; Röder, K.; Gronenberg, O.; Vahl, A.; Polonskyi, O.; Strunskus, T.; Rubahn, H.-G.; Kienle, L.; Adam, J.; Fiutowski, J.; et al. Cauliflower-like CeO2–TiO2 hybrid nanostructures with extreme photocatalytic and self-cleaning properties. Nanoscale 2019, 11, 9840–9844. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Nguyen-Phan, T.-D.; Johnston-Peck, A.C.; Barrio, L.; Sallis, S.; Arena, D.A.; Kundu, S.; Xu, W.; Piper, L.F.J.; Stach, E.A.; et al. Hierarchical Heterogeneity at the CeOx–TiO2 Interface: Electronic and Geometric Structural Influence on the Photocatalytic Activity of Oxide on Oxide Nanostructures. J. Phys. Chem. C 2015, 119, 2669–2679. [Google Scholar] [CrossRef]
- Johnston-Peck, A.C.; Senanayake, S.D.; Plata, J.J.; Kundu, S.; Xu, W.; Barrio, L.; Graciani, J.; Sanz, J.F.; Navarro, R.M.; Fierro, J.L.G.; et al. Nature of the Mixed-Oxide Interface in Ceria–Titania Catalysts: Clusters, Chains, and Nanoparticles. J. Phys. Chem. C 2013, 117, 14463–14471. [Google Scholar] [CrossRef]
- Feist, T.P.; Davies, P.K. The soft chemical synthesis of TiO2 (B) from layered titanates. J. Solid State Chem. 1992, 101, 275–295. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Lan, Y.; Gao, X.P.; Ringer, S.P.; Zheng, Z.F.; Song, D.Y.; Zhao, J.C. Phase Transition between Nanostructures of Titanate and Titanium Dioxides via Simple Wet-Chemical Reactions. J. Am. Chem. Soc. 2005, 127, 6730–6736. [Google Scholar] [CrossRef]
- Kiatkittipong, K.; Scott, J.; Amal, R. Hydrothermally Synthesized Titanate Nanostructures: Impact of Heat Treatment on Particle Characteristics and Photocatalytic Properties. ACS Appl. Mater. Interfaces 2011, 3, 3988–3996. [Google Scholar] [CrossRef]
- Qamar, M.; Yoon, C.R.; Oh, H.J.; Kim, D.H.; Jho, J.H.; Lee, K.S.; Lee, W.J.; Lee, H.G.; Kim, S.J. Effect of post treatments on the structure and thermal stability of titanate nanotubes. Nanotechnology 2006, 17, 5922. [Google Scholar] [CrossRef]
- Opra, D.P.; Gnedenkov, S.V.; Sokolov, A.A.; Podgorbunsky, A.B.; Ustinov, A.Y.; Mayorov, V.Y.; Kuryavyi, V.G.; Sinebryukhov, S.L. Vanadium-doped TiO2-B/anatase mesoporous nanotubes with improved rate and cycle performance for rechargeable lithium and sodium batteries. J. Mater. Sci. Technol. 2020, 54, 181–189. [Google Scholar] [CrossRef]
- Uesugi, Y.; Nagakawa, H.; Nagata, M. Highly Efficient Photocatalytic Degradation of Hydrogen Sulfide in the Gas Phase Using Anatase/TiO(2)(B) Nanotubes. ACS Omega 2022, 7, 11946–11955. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Q.; Ng, A.M.C.; Djurišić, A.B.; Xie, M.; Liao, C.; Shih, K.; Vranješ, M.; Nedeljković, J.M.; Deng, Z. In situ synthesis of TiO2(B) nanotube/nanoparticle composite anode materials for lithium ion batteries. Nanotechnology 2015, 26, 425403. [Google Scholar] [CrossRef]
- Huang, J.P.; Yuan, D.D.; Zhang, H.Z.; Cao, Y.L.; Li, G.R.; Yang, H.X.; Gao, X.P. Electrochemical sodium storage of TiO2(B) nanotubes for sodium ion batteries. RSC Adv. 2013, 3, 12593–12597. [Google Scholar] [CrossRef]
- Liu, Z.; Andreev, Y.G.; Robert Armstrong, A.; Brutti, S.; Ren, Y.; Bruce, P.G. Nanostructured TiO2(B): The effect of size and shape on anode properties for Li-ion batteries. Prog. Nat. Sci. Mater. Int. 2013, 23, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, Y.; Yu, L.; Wu, N.-L.; Huang, H.; Wei, M. TiO2-B nanowires via topological conversion with enhanced lithium-ion intercalation properties. J. Mater. Chem. A 2019, 7, 3842–3847. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Armstrong, G.; Canales, J.; Bruce, P.G. TiO2-B Nanowires. Angew. Chem. Int. Ed. 2004, 43, 2286–2288. [Google Scholar] [CrossRef]
- Umek, P.; Bittencourt, C.; Guttmann, P.; Gloter, A.; Škapin, S.D.; Arčon, D. Mn2+ Substitutional Doping of TiO2 Nanoribbons: A Three-Step Approach. J. Phys. Chem. C 2014, 118, 21250–21257. [Google Scholar] [CrossRef]
- Kolen’ko, Y.V.; Kovnir, K.; Gavrilov, A.I.; Garshev, A.V.; Frantti, J.; Lebedev, O.I.; Churagulov, B.R.; van Tendeloo, G.; Yoshimura, M.J.T.j.o.p.c.B. Hydrothermal synthesis and characterization of nanorods of various titanates and titanium dioxide. J. Phys. Chem. B 2006, 110, 4030–4038. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Li, J.; Yu, Y.; Li, J. From titanates to TiO2 nanostructures: Controllable synthesis, growth mechanism, and applications. Sci. China Chem. 2012, 55, 2334–2345. [Google Scholar] [CrossRef]
- Wen, P.; Ishikawa, Y.; Itoh, H.; Feng, Q. Topotactic Transformation Reaction from Layered Titanate Nanosheets into Anatase Nanocrystals. J. Phys. Chem. C 2009, 113, 20275–20280. [Google Scholar] [CrossRef]
- Akimoto, J.; Chiba, K.; Kijima, N.; Hayakawa, H.; Hayashi, S.; Gotoh, Y.; Idemoto, Y. Soft-Chemical Synthesis and Electrochemical Property of H2Ti12O25 as a Negative Electrode Material for Rechargeable Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, A546. [Google Scholar] [CrossRef]
- Sluban, M.; Umek, P. Role of Water in the Transformation of Protonated Titanate Nanoribbons to Anatase Nanoribbons. J. Phys. Chem. C 2019, 123, 23747–23757. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Su, K.; Cheng, B. Excellent performance of TiO2(B) nanotubes in selective transesterification of DMC with phenol derivatives. Chem. Eng. J. 2016, 301, 12–18. [Google Scholar] [CrossRef]
- Viana, B.C.; Ferreira, O.P.; Souza Filho, A.G.; Rodrigues, C.M.; Moraes, S.G.; Mendes Filho, J.; Alves, O.L. Decorating Titanate Nanotubes with CeO2 Nanoparticles. J. Phys. Chem. C 2009, 113, 20234–20239. [Google Scholar] [CrossRef]
- Marques, T.M.F.; Ferreira, O.P.; da Costa, J.A.P.; Fujisawa, K.; Terrones, M.; Viana, B.C. Study of the growth of CeO2 nanoparticles onto titanate nanotubes. J. Phys. Chem. Solids 2015, 87, 213–220. [Google Scholar] [CrossRef]
- Gu, X.; Chen, F.; Zhao, B.; Zhang, J. Photocatalytic reactivity of Ce-intercalated layered titanate prepared with a hybrid method based on ion-exchange and thermal treatment. Superlattices Microstruct. 2011, 50, 107–118. [Google Scholar] [CrossRef]
- De Santis, S.; Sotgiu, G.; Porcelli, F.; Marsotto, M.; Iucci, G.; Orsini, M. A Simple Cerium Coating Strategy for Titanium Oxide Nanotubes’ Bioactivity Enhancement. Nanomaterials 2021, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, L.; Chen, Y.; Fang, M.; Zhang, J.; Wang, H. Highly Efficient, Irreversible and Selective Ion Exchange Property of Layered Titanate Nanostructures. Adv. Funct. Mater. 2012, 22, 835–841. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Hsu, W.P.; Ronnquist, L.; Matijevic, E. Preparation and properties of monodispersed colloidal particles of lanthanide compounds. 2. Cerium(IV). Langmuir 1988, 4, 31–37. [Google Scholar] [CrossRef]
- Aubriet, F.; Gaumet, J.-J.; de Jong, W.A.; Groenewold, G.S.; Gianotto, A.K.; McIlwain, M.E.; Van Stipdonk, M.J.; Leavitt, C.M. Cerium Oxyhydroxide Clusters: Formation, Structure, and Reactivity. J. Phys. Chem. A 2009, 113, 6239–6252. [Google Scholar] [CrossRef]
- Ikeda-Ohno, A.; Hennig, C.; Weiss, S.; Yaita, T.; Bernhard, G. Hydrolysis of Tetravalent Cerium for a Simple Route to Nanocrystalline Cerium Dioxide: An In Situ Spectroscopic Study of Nanocrystal Evolution. Chem. A Eur. J. 2013, 19, 7348–7360. [Google Scholar] [CrossRef]
- Briois, V.; Williams, C.E.; Dexpert, H.; Villain, F.; Cabane, B.; Deneuve, F.; Magnier, C. Formation of solid particles by hydrolysis of cerium (IV) sulphate. J. Mater. Sci. 1993, 28, 5019–5031. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Krüger, P.; Sluban, M.; Umek, P.; Guttmann, P.; Bittencourt, C. Chemical Bond Modification upon Phase Transformation of TiO2 Nanoribbons Revealed by Nanoscale X-ray Linear Dichroism. J. Phys. Chem. C 2017, 121, 17038–17042. [Google Scholar] [CrossRef]
- López, T.; Rojas, F.; Alexander-Katz, R.; Galindo, F.; Balankin, A.; Buljan, A. Porosity, structural and fractal study of sol–gel TiO2–CeO2 mixed oxides. J. Solid State Chem. 2004, 177, 1873–1885. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, J.; Sun, Y.; Li, K.; Shang, T.; Wan, Y. Recent advances and perspectives of CeO2-based catalysts: Electronic properties and applications for energy storage and conversion. Front. Chem. 2022, 10, 1089708. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 Photocatalysis: Fundamentals and Applications; BKC: Mumbai, India, 1999. [Google Scholar]
- Singh, M.; Taele, B.M.; Goyal, M. Modeling of size and shape dependent band gap, dielectric constant and phonon frequency of semiconductor nanosolids. Chin. J. Phys. 2021, 70, 26–36. [Google Scholar] [CrossRef]
- Umek, P.; Korošec, R.C.; Jančar, B.; Dominko, R.; Arčon, D. The Influence of the Reaction Temperature on the Morphology of Sodium Titanate 1D Nanostructures and Their Thermal Stability. J. Nanosci. Nanotechnol. 2007, 7, 3502–3508. [Google Scholar] [CrossRef]
- Fortner, J.A.; Buck, E.C. The chemistry of the light rare-earth elements as determined by electron energy loss spectroscopy. Appl. Phys. Lett. 1996, 68, 3817–3819. [Google Scholar] [CrossRef]
- Song, K.; Schmid, H.; Srot, V.; Gilardi, E.; Gregori, G.; Du, K.; Maier, J.; van Aken, P.A. Cerium reduction at the interface between ceria and yttria-stabilised zirconia and implications for interfacial oxygen non-stoichiometry. APL Mater. 2014, 2, 032104. [Google Scholar] [CrossRef]
- Kirsch, H. Physics of Minerals and Inorganic Materials. Von, A.S. Marfunin. Springer-Verlag Berlin–Heidelberg–New York 1979, 340 S. mit 138 Abb. u. 50 Tab., XII, Ln. 98,– DM. Mater. Corros. 1980, 31, 155–156. [Google Scholar] [CrossRef]
- Casari, B.M.; Langer, V. Two Ce(SO4)2⋅4H2O polymorphs: Crystal structure and thermal behavior. J. Solid State Chem. 2007, 180, 1616–1622. [Google Scholar] [CrossRef]
- Strydom, C.A.; Van Vuuren, C.P.J. The thermal decomposition of cerium (III) nitrate. J. Therm. Anal. 1987, 32, 157–160. [Google Scholar] [CrossRef]





| Sample Labels | Precursor | Transformation Environment | T (°C) | Phase Composition | Ce CONTENT a (wt.%) | Ce Oxidation State b | Band Gap (eV) |
|---|---|---|---|---|---|---|---|
| Ce4+-HTiNRs | H2Ti3O7 NRs | DI water c | 100 | H2Ti3O7 | 3.4 | +3, +4 (80%) | 3.56 |
| Ce4+-620 °C | Ce4+-HTiNRs | static air | 620 | TiO2-B | 3.9 | +3, +4 (75%) | 3.30 |
| Ce4+-750 °C | Ce4+-HTiNRs | static air | 750 | TiO2-B, anatase | 3.9 | +3, +4 (70%) | 3.17 |
| Ce4+-860 °C | Ce4+-HTiNRs | static air | 860 | anatase | 3.8 | +3, +4 (50%) | 3.27 |
| Ce4+-960 °C | Ce4+-HTiNRs | static air | 960 | anatase, rutile | 3.7 | +3, +4 (50%) | 3.19 |
| Ce3+-HTiNRs | H2Ti3O7 NRs | DI water b | 100 | H2Ti3O7 | 0.2 | +3, +4 | 3.53 |
| Ce3+-620 °C | Ce3+-HTiNRs | static air | 620 | TiO2-B, anatase | 0.3 | +3, +4 | 3.40 |
| Ce3+-750 °C | Ce3+-HTiNRs | static air | 750 | anatase, traces of TiO2-B | 0.3 | +3, +4 | 3.36 |
| Ce3+-860 °C | Ce3+-HTiNRs | static air | 860 | anatase | 0.3 | +3, +4 | 3.34 |
| Ce3+-960 °C | Ce3+-HTiNRs | static air | 960 | anatase, rutile | 0.4 | +3, +4 | 3.31 |
| HTiNRs | / | / | / | H2Ti3O7 | / | / | 3.46 |
| d p-620 °C | H2Ti3O7 NRs | static air | 620 | anatase, traces of TiO2-B | / | / | 3.31 |
| p-750 °C | H2Ti3O7 NRs | static air | 750 | anatase | / | / | 3.28 |
| p-860 °C | H2Ti3O7 NRs | static air | 860 | anatase | / | / | 3.29 |
| p-960 °C | H2Ti3O7 NRs | static air | 960 | anatase, rutile | / | / | 3.01 |
| Sample | wt.% Rutile |
|---|---|
| Ce4+-960 °C | 31 |
| Ce3+-960 °C | 17 |
| p-960 °C | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umek, P.; Dürrschnabel, M.; Molina-Luna, L.; Škapin, S.; Korošec, R.C.; Bittencourt, C. The Role of Cerium Valence in the Conversion Temperature of H2Ti3O7 Nanoribbons to TiO2-B and Anatase Nanoribbons, and Further to Rutile. Molecules 2023, 28, 5838. https://doi.org/10.3390/molecules28155838
Umek P, Dürrschnabel M, Molina-Luna L, Škapin S, Korošec RC, Bittencourt C. The Role of Cerium Valence in the Conversion Temperature of H2Ti3O7 Nanoribbons to TiO2-B and Anatase Nanoribbons, and Further to Rutile. Molecules. 2023; 28(15):5838. https://doi.org/10.3390/molecules28155838
Chicago/Turabian StyleUmek, Polona, Michael Dürrschnabel, Leopoldo Molina-Luna, Srečo Škapin, Romana Cerc Korošec, and Carla Bittencourt. 2023. "The Role of Cerium Valence in the Conversion Temperature of H2Ti3O7 Nanoribbons to TiO2-B and Anatase Nanoribbons, and Further to Rutile" Molecules 28, no. 15: 5838. https://doi.org/10.3390/molecules28155838