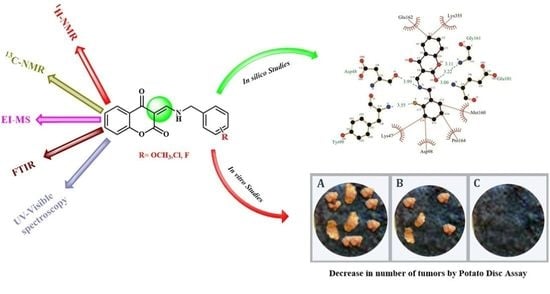
In Silico and In Vitro Studies of 4-Hydroxycoumarin-Based Heterocyclic Enamines as Potential Anti-Tumor Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Compounds (4a–4i)
2.2. Optimization of Reaction Conditions
2.3. Anti-Tumor Activity
2.4. Molecular Docking Studies
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Substituted 3-((Benzylamino)methylene)-3H-chromene-2,4-dione (4a–4i)
3.3. Proposed Mechanism of Reaction
3.4. Potato Disc Tumor Assay
3.5. Molecular Docking Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Huang, Q.; Wang, X.; Chen, A.; Zhang, H.; Yu, Q.; Shen, C.; Awadasseid, A.; Zhao, X.; Xiong, X.; Wu, Y.; et al. Design, synthesis and anti-tumor activity of novel benzothiophenonaphthalimide derivatives targeting mitochondrial DNA (mtDNA) G-quadruplex. Biochem. Pharmacol. 2022, 201, 115062. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. World cancer report 2014. Geneva, Switzerland: World Health Organization, international agency for research on cancer, WHO Press, 2015. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448. [Google Scholar] [CrossRef]
- Subramanian, A.; John, A.; Vellayappan, M.; Balaji, A.; Jaganathan, S.; Supriyanto, E.; Yusof, M. Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Adv. 2015, 5, 35608–35621. [Google Scholar] [CrossRef]
- Zaki, M.; Hairat, S.; Aazam, E.S. Scope of organometallic compounds based on transition metal-arene systems as anticancer agents: Starting from the classical paradigm to targeting multiple strategies. RSC Adv. 2019, 9, 3239–3278. [Google Scholar] [CrossRef]
- Lv, W.; Fu, B.; Li, M.; Kang, Y.; Bai, S.; Lu, C. Determination of IC 50 values of anticancer drugs on cells by D2O–single cell Raman spectroscopy. Chem. Comm. 2022, 58, 2355–2358. [Google Scholar] [CrossRef]
- Suroowan, S.; Mahomoodally, M.F. Tradition Meets Innovation: Herbal Medicine as a Sustainable Source of Anticancer Agents. In Urban Health Risk and Resilience in Asian Cities; Springer: Berlin/Heidelberg, Germany, 2020; pp. 367–387. [Google Scholar]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of metal-based drugs according to their mechanisms of action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef]
- Gailer, J. Improving the safety of metal-based drugs by tuning their metabolism with chemoprotective agents. J. Inorg. Biochem. 2018, 179, 154–157. [Google Scholar] [CrossRef]
- Vaseghi, S.; Yousefi, M.; Shokrzadeh, M.; Hossaini, Z.; Hosseini-Khah, Z.; Emami, S. Synthesis, computational study and cytotoxicity of 4-hydroxycoumarin-derived imines/enamines. Mol. Divers. 2021, 25, 1011–1024. [Google Scholar] [CrossRef]
- Ibrahim, M.; Latif, A.; Ahmad, M.; Ahmad, S.; Ali, A.; Siddique, A.B.; Saadiq, M.; Akbar, N.; Khan, A.; Al-Harrasi, A. Sulfonylbis (acylhydrazones) as anticholinesterase inhibitors: Synthesis, in vitro biological evaluation and computational studies. J. Mol. Struct. 2022, 1252, 132215. [Google Scholar] [CrossRef]
- Malik, A.N.; Kuznetsov, A.; Ali, A.; Ashfaq, M.; Tahir, M.N.; Siddique, A. Imine-based Zwitterion: Synthesis, single-crystal characterization, and computational investigation. J. Mol. Struct. 2022, 1253, 132237. [Google Scholar] [CrossRef]
- Uroos, M.; Javaid, A.; Bashir, A.; Tariq, J.; Khan, I.H.; Naz, S.; Fatima, S.; Sultan, M. Green synthesis of coumarin derivatives using Brønsted acidic pyridinium based ionic liquid [MBSPy][HSO4] to control an opportunistic human and a devastating plant pathogenic fungus Macrophomina phaseolina. RSC Adv. 2022, 12, 23963–23972. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F. Natural coumarins: Exploring the pharmacological complexity and underlying molecular mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ham, J.; Hong, T.; Song, G.; Lim, W. Fraxetin suppresses cell proliferation and induces apoptosis through mitochondria dysfunction in human hepatocellular carcinoma cell lines Huh7 and Hep3B. Pharmaceutics 2021, 13, 112. [Google Scholar] [CrossRef]
- Konarska-Bajda, K.; Ceranowicz, P.; Cieszkowski, J.; Ginter, G.; Stempniewicz, A.; Gałązka, K.; Warzecha, Z. Administration of Warfarin Inhibits the Development of Cerulein-Induced Edematous Acute Pancreatitis in Rats. Biomolecules 2023, 13, 948. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Goteri, G.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D. The Role of NQO1 in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 7839. [Google Scholar] [CrossRef]
- Ma, Z.; Peng, L.; Chu, W.; Wang, P.; Fu, Y. Osthole Alleviates D-Galactose-Induced Liver Injury In Vivo via the TLR4/MAPK/NF-κB Pathways. Molecules 2023, 28, 443. [Google Scholar] [CrossRef]
- Youness, R.A.; Al-Mahallawi, A.M.; Mahmoud, F.H.; Atta, H.; Braoudaki, M.; Fahmy, S.A. Oral Delivery of Psoralidin by Mucoadhesive Surface-Modified Bilosomes Showed Boosted Apoptotic and Necrotic Effects against Breast and Lung Cancer Cells. Polymers 2023, 15, 1464. [Google Scholar] [CrossRef]
- Magoo, D.; Aggarwal, K.; Gupta, S.; Meena, K. Enamines and their variants as intermediates for synthesis of aza-heterocycles with applications in MCRs. Tetrahedron 2022, 103, 132545. [Google Scholar] [CrossRef]
- Vodolazhenko, M.A.; Gorobets, N.Y.; Zhikol, O.A.; Desenko, S.M.; Shishkin, O.V. A quantum chemical approach towards understanding stability and tautomerism of 2-imino-2H-pyran derivatives. RSC Adv. 2016, 6, 52201–52211. [Google Scholar] [CrossRef]
- Farid, S.M.; Seifinoferest, B.; Gholamhosseyni, M.; Larijani, B.; Mahdavi, M. Modern metal-catalyzed and organocatalytic methods for synthesis of coumarin derivatives: A review. Org. Biomol. Chem. 2022, 20, 4846–4883. [Google Scholar] [CrossRef] [PubMed]
- Holiyachi, M.; Shastri, S.L.; Chougala, B.M.; Naik, N.S.; Pawar, V.; Shastri, L.A.; Sunagar, V.A. Design and synthesis of new series of dipyrromethane-coumarin and porphyrin-coumarin derivatives: Excellent anticancer agents. J. Mol. Struct. 2021, 1237, 130424. [Google Scholar] [CrossRef]
- Premnath, P.N.; Craig, S.N.; Liu, S.; Anderson, E.L.; Grigoroudis, A.I.; Kontopidis, G.; McInnes, C. Iterative conversion of cyclin binding groove peptides into druglike CDK inhibitors with antitumor activity. J. Med. Chem. 2015, 58, 433–442. [Google Scholar] [CrossRef]
- D’costa, M.; Bothe, A.; Das, S.; Kumar, S.U.; Gnanasambandan, R.; Doss, C.G.P. CDK regulators—Cell cycle progression or apoptosis—Scenarios in normal cells and cancerous cells. Adv. Protein Chem. Struct. 2023, 135, 125–177. [Google Scholar]
- Olyaei, A.; Javarsineh, S.; Sadeghpour, M. Green synthesis and Z/E-isomerization of novel coumarin enamines induced by organic solvents. Chem. Heterocycl. Compd. 2018, 54, 934–939. [Google Scholar] [CrossRef]
- Mpitimpiti, A.N.; Petzer, J.P.; Petzer, A.; Jordaan, J.H.; Lourens, A.C. Synthesis and evaluation of chromone derivatives as inhibitors of monoamine oxidase. Mol. Divers. 2019, 23, 897–913. [Google Scholar] [CrossRef]
- Coker, P.; Radecke, J.; Guy, C.; Camper, N.D. Potato disc tumor induction assay: A multiple mode of drug action assay. Phytomedicine 2003, 10, 133–138. [Google Scholar] [CrossRef]
- McLaughlin, J.L.; Hostettmann, K. Methods in plant biochemistry. Assays Bioactivity 1991, 6, 1–33. [Google Scholar]
- Binns, A.N.; Thomashow, M.F. Cell biology of Agrobacterium infection and transformation of plants. Annu. Rev. Microbiol. 1988, 42, 575–606. [Google Scholar] [CrossRef]
- McLaughlin, J.L.; Rogers, L.L.; Anderson, J.E. The use of biological assays to evaluate botanicals. Drug Inf. J. 1998, 32, 513–524. [Google Scholar] [CrossRef]
- Agrwal, A.; Saini, R.; Bhandri, S.; Verma, S.; Srivastava, P.; Prakash, O. Synthesis, ADMET, drug likeness and in silico activities of benzimidazole derivative. Mater. Today Proc. 2022, 67, 598–604. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ahmad, S.; Shaheen, M.A.; Ali, A.; Tahir, M.N.; Vieira, L.C.; Muhammad, S.; Siddeeg, S.M. Synthesis, antimicrobial potential and computational studies of crystalline 4-bromo-2-(1,4,5-triphenyl-1H-imidazole-2-yl)phenol and its metal complexes. CrystEngComm 2022, 24, 8237–8247. [Google Scholar] [CrossRef]
- Dannappel, M.V.; Sooraj, D.; Loh, J.J.; Firestein, R. Molecular and in vivo functions of the CDK8 and CDK19 kinase modules. Front. Cell Dev. Biol. 2019, 6, 171. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Martinez, C.; Gelbert, L.M.; Lallena, M.J.; de Dios, A. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs. Bioorg. Med. Chem. Lett. 2015, 25, 3420–3435. [Google Scholar] [CrossRef]
- Hamdi, M.; Granier, P.; Sakellariou, R.; Spéziale, V. Reaction of amines on 3-ureidomethylenecoumarins. A new route to N-(methylene-4-oxocoumarinyl) amines. J. Heterocycl. Chem. 1993, 30, 1155–1157. [Google Scholar] [CrossRef]
- Hamdi, M.; Sakellariou, R.; Speziale, V. New Condensation Products of Diamines with 3-Ureidomethylenecoumarin. Org. Prep. Proced. Int. 1995, 27, 487–492. [Google Scholar] [CrossRef]
- Chun, R. Cancer Chemotherapy. In Withrow & MacEwen’s Small Animal Clinical Oncology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 163–192. [Google Scholar]
- Krieger, E.; Vriend, G. YASARA View—Molecular graphics for all devices—From smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.F.u.; Akhter, S.; Batool, A.I.; Selamoglu, Z.; Sevindik, M.; Eman, R.; Mustaqeem, M.; Akram, M.S.; Kanwal, F.; Lu, C.; et al. Effectiveness of Natural Antioxidants against SARS-CoV-2? Insights from the In-Silico World. Antibiotics 2021, 10, 1011. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery; ACS Publications: Washington, DC, USA, 2011. [Google Scholar]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]






| Sr. No. | Solvents | Catalysts | Reaction Conditions | Yield % |
|---|---|---|---|---|
| 1 | MeOH | - | Reflux, 3 h | 80 |
| 2 | EtOH | - | Reflux, 3 h | 75 |
| 3 | THF | - | Reflux, 3 h | 65 |
| 4 | CHCl3 | - | Reflux, 3 h | 64 |
| 5 | 2-BuOH | - | Reflux, 3 h | 94 |
| 6 | 2-BuOH | - | Reflux, 6 h | 78 |
| 7 | 2-BuOH | AcOH | Reflux, 3 h | 55 |
| 8 | 2-BuOH | ZnCl2 | Reflux, 3 h | 72 |
| 9 | 2-BuOH | AlCl3 | Reflux, 3 h | 74 |
| 10 | 2-BuOH | (CH3COO)2Pb | Reflux, 3 h | 71 |
| 11 | 2-BuOH | Net3 | Reflux, 3 h | 60 |
| 12 | 2-BuOH | HCl | Reflux, 3 h | 52 |
| Sr. No. | Compound | Protein | Binding Energy (kcal/mol) | Dissociation Constant (nM) | Main Contacting Amino Acid Residues |
|---|---|---|---|---|---|
| 1 | 4a | CDK-8 | −9.22 | 175.54 | Lys-47, Asp-98, Gly-161 & Glu- 101 |
| 2 | 4b | −8.45 | 89.46 | Lys-47, Tyr-99, Gly-161 & Glu-101 | |
| 3 | 4c | −9.41 | 444.39 | His-154, Pro-194 & Phe-195, | |
| 4 | 4d | −8.87 | 194.89 | Lys-47, Asp-48, Tyr-99, & Gly-161 | |
| 5 | 4e | −9.33 | 192.9 | Lys-47, Tyr-99, Gly-161 & Glu-165 | |
| 6 | 4f | −8.66 | 83.5 | Tyr-153, Tyr-156, Arg-157 & Pro-158 | |
| 7 | 4g | −9.53 | 106.69 | Lys-47, Asp-98, Tyr-99 & Glu- 101 | |
| 8 | 4h | −9.52 | 104.28 | Trp-6, Glu-98, His-154 & Pro-194 | |
| 9 | 4i | −8.53 | 98.42 | Phe-5, Glu-98, His-154 & Phe-195 | |
| 10 | Vinblastine | −9.11 | 3170 | Asp-46, Lys-47, Gly-161 & Lys-355 | |
| 11 | 5XG | −9.18 | 185.8 | Val-27, Ala-100, Asp-173 & Arg-356 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assad, M.; Paracha, R.N.; Siddique, A.B.; Shaheen, M.A.; Ahmad, N.; Mustaqeem, M.; Kanwal, F.; Mustafa, M.Z.U.; Rehman, M.F.u.; Fatima, S.; et al. In Silico and In Vitro Studies of 4-Hydroxycoumarin-Based Heterocyclic Enamines as Potential Anti-Tumor Agents. Molecules 2023, 28, 5828. https://doi.org/10.3390/molecules28155828
Assad M, Paracha RN, Siddique AB, Shaheen MA, Ahmad N, Mustaqeem M, Kanwal F, Mustafa MZU, Rehman MFu, Fatima S, et al. In Silico and In Vitro Studies of 4-Hydroxycoumarin-Based Heterocyclic Enamines as Potential Anti-Tumor Agents. Molecules. 2023; 28(15):5828. https://doi.org/10.3390/molecules28155828
Chicago/Turabian StyleAssad, Mediha, Rizwan Nasir Paracha, Abu Bakar Siddique, Muhammad Ashraf Shaheen, Nadeem Ahmad, Muhammad Mustaqeem, Fariha Kanwal, Muhammad Zia Ul Mustafa, Muhammad Fayyaz ur Rehman, Sumaya Fatima, and et al. 2023. "In Silico and In Vitro Studies of 4-Hydroxycoumarin-Based Heterocyclic Enamines as Potential Anti-Tumor Agents" Molecules 28, no. 15: 5828. https://doi.org/10.3390/molecules28155828