Enhanced Oxidation of p-Toluidine Using Supported Zeolite Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dynamic Light Scattering (DLS) Measurements
2.2. X-ray Diffraction (XRD) Analysis
2.3. Scanning Electron Microscopy (SEM) Analysis
2.4. Transmission Electron Microscopy (TEM) Analysis
2.5. Surface Area and Pore Size Analysis
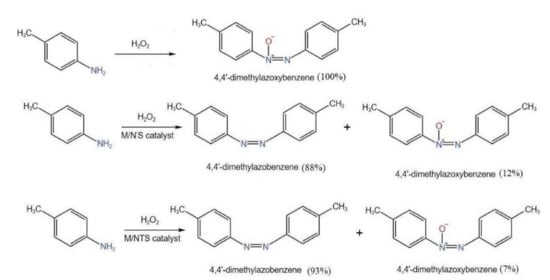
2.6. Catalytic Activity Studies
2.6.1. Effect of Mole Ratio
2.6.2. Effect of Catalyst Quantity
2.6.3. Reaction Mechanism
2.6.4. Reusability of Catalyst
2.6.5. Comparison of Catalysts
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Magnetite Nanoparticles
3.3. Synthesis of Nanocrytalline TS-1
3.4. Synthesis of Nanocrytalline Silicalite-1
3.5. Synthesis of Magnetite Supported Nanocrystalline Zeolite
3.6. Characterization Methods
3.7. Catalytic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nemeth, L.; Bare, S.R. Science and technology of framework metal-containing zeotype catalysts. Adv. Catal. 2014, 57, 1–97. [Google Scholar]
- Firouzabadi, H.; Jafari, A.A. Heteropolyacids, their salts and polyoxometalates as heterogenous, efficient and eco-friendly catalysts in organic reactions: Some recent advances. J. Iran. Chem. Soc. 2005, 2, 85–114. [Google Scholar] [CrossRef]
- Kozhevnikov, V.; Derouane, E. (Eds.) Catalysts for Fine Chemical Synthesis. Catalysis by Polyoxometalates 2; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Peter, M.; de Jongh, P.E.; de Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Ding, K. Self-Supported catalysts. Chem. Rev. 2009, 109, 322–359. [Google Scholar] [CrossRef]
- Centi, G.; Ciambelli, P.; Perathoner, S.; Russo, P. Environmental catalysis: Trends and outlook. Catal. Today 2002, 75, 3–15. [Google Scholar] [CrossRef]
- Sunitha, K.; Richa, S.; Manisha, S. Electrochemical studies of toluidines with special reference of effect of pH, scan rate. Int. J. Sci. Eng. Appl. Sci. 2016, 2, 320–323. [Google Scholar]
- Margetic, D.; Štrukil, V. Mechanochemical Organic Synthesis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 296–297. [Google Scholar]
- Kametani, T.; Ogasawara, K. The Oxidation of p-Toluidine with Potassium Ferricyanide in Liquid Ammonia. Chem. Pharm. Bull. 1968, 16, 1843–1845. [Google Scholar] [CrossRef] [Green Version]
- Börnstein, E. Fine Umalagerung in der Chinongruppe. Chem. Ber. 1910, 43, 2380–2384. [Google Scholar] [CrossRef] [Green Version]
- Holland, V.R.; Saunders, B.C. Studies in peroxidase action—XX: The oxidation of a mixture of p-toluidine and 4-chloroaniline. Tetrahedron 1969, 25, 4153–4160. [Google Scholar] [CrossRef]
- John Plater, M.; William, T.A. Harrison Barsilowsky’s base and Perkin’s base: Two products from the oxidation of p-toluidine. J. Chem. Res. 2014, 38, 351–355. [Google Scholar] [CrossRef]
- Ritu Singh Kinetics of oxidation of o-toluidine by potassium dichromate. Int. J. Sci. Res. Publ. 2016, 6, 234–237.
- Pausacker, K.H.; Scroggie, J.G. Oxidations with lead tetra-acetate. Part II. The oxidation of primary aromatic amines. J. Chem. Soc. 1954, 4003–4006. [Google Scholar] [CrossRef]
- Kaushik, R.D.; Kumar, V.; Arya, R.K. Darshu Singh Periodate oxidation of o-toluidine in acetone-water medium: A kinetic and mechanistic study. Asian J. Chem. 2000, 12, 1123–1128. [Google Scholar]
- Wang, J.; Park, J.-N.; Jeong, H.-C.; Choi, K.-S.; Wei, X.-Y.; Hong, S.-I.; Lee, C.W. Cu2+-Exchanged Zeolites as Catalysts for Phenol Hydroxylation with Hydrogen Peroxide. Energy Fuels 2004, 18, 470–476. [Google Scholar] [CrossRef]
- Zhu, Z.; Espenson, J.H. Kinetics and Mechanism of Oxidation of Anilines by Hydrogen Peroxide as Catalyzed by Methylrhenium Trioxide. J. Org. Chem. 1995, 60, 1326–1332. [Google Scholar] [CrossRef]
- Yu, R.; Xiao, F.; Wang, D.; Sun, J.; Liu, Y.; Pang, G.; Feng, S.; Qiu, S.; Xu, R.; Fang, C. Catalytic performance in phenol hydroxylation by hydrogen peroxide over a catalyst of V–Zr–O complex. Catal. Today 1999, 51, 39–46. [Google Scholar] [CrossRef]
- Sun, J.; Meng, X.; Shi, Y.; Wang, R.; Feng, S.; Jiang, D.; Xu, R.; Xiao, F. A Novel Catalyst of Cu–Bi–V–O Complex in Phenol Hydroxylation with Hydrogen Peroxide. J. Catal. 2000, 193, 199–206. [Google Scholar] [CrossRef]
- Dubey, A.; Rives, V.; Kannan, S. Catalytic hydroxylation of phenol over ternary hydrotalcites containing Cu, Ni and Al. J. Mol. Catal. A: Chem. 2002, 181, 151–160. [Google Scholar] [CrossRef]
- Lehman, S.E.; Larsen, S.C. Zeolite and mesoporous silica nanomaterials: Greener syntheses, environmental applications and biological toxicity. Environ. Sci. Nano 2014, 1, 200–213. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; Wiley-Interscience: New York, NY, USA, 1974. [Google Scholar]
- Valtchev, V.; Tosheva, L. Porous Nanosized Particles: Preparation, Properties and Applications. Chem. Rev. 2013, 113, 6734–6760. [Google Scholar] [CrossRef] [PubMed]
- Tosheva, L.; Valtchev, V.P. Nanozeolites: Synthesis, Characterisation and Applications. Chem. Mater 2005, 17, 2494–2513. [Google Scholar] [CrossRef]
- Li, Z.X.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.T.; Lin, V.S.Y. Synthesis and functionalisation of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, C.; Wang, L.; Wang, L.; Xiao, F.-S. Zeolite Fixed Metal Nanoparticles: New Perspective in Catalysis. Acc. Chem. Res. 2021, 54, 2579–2590. [Google Scholar]
- Wróblewska, A.; Makuch, E.; Miądlicki, P. The studies on the limonene oxidation over the microporous TS-1 catalyst. Catal. Today 2016, 268, 121–129. [Google Scholar] [CrossRef]
- Yuan, W.W.; Yuan, P.; Liu, D.; Yu, W.B.; Laipan, M.W.; Deng, L.L.; Chen, F.R. In situ hydrothermal synthesis of a novel hierarchically porous TS-1/modified-diatomite composite for methylene blue (MB) removal by the synergistic effect of adsorption and photocatalysis. J. Colloid Interface Sci. 2016, 462, 191–199. [Google Scholar]
- Shamzhy, M.; Gil, B.; Opanasenko, M.; Roth, W.J.; Čejka, J. MWW and MFI Frameworks as Model Layered Zeolites: Structures, Transformations, Properties, and Activity. ACS Catal. 2021, 11, 2366–2396. [Google Scholar]
- Zhang, T.; Zuo, Y.; Liu, M.; Song, C.; Guo, X. Synthesis of Titanium Silicalite-1 with High Catalytic Performance for 1-Butene Epoxidation by Eliminating the Extra framework Ti. ACS Omega 2016, 1, 1034–1040. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H.; Yi, C. Preparation of photo-Fenton heterogeneous catalyst (Fe-TS-1 zeolite) and its application in typical azo dye decoloration. J. Photochem. Photobiol. A Chem. 2018, 356, 138–149. [Google Scholar] [CrossRef]
- Zhen, C.; Lianlin, Z.; Yunkai, Y.; Dongxu, L.; Nan, F.; Yueming, L.; Mingyuan, H. Molecular traffic control for catalytic oxidation reaction in TS-1 zeolite. Microporous Mesoporous Mater. 2022, 332, 111715. [Google Scholar]
- Dong, C.Y.; Li, Y.L.; Cheng, D.Y.; Zhang, M.T.; Liu, J.J.; Wang, Y.G.; Xiao, D.Q.; Ma, D. Supported Metal Clusters: Fabrication and Application in Heterogeneous Catalysis. ACS Catal. 2020, 10, 11011–11045. [Google Scholar]
- Li, Z.; Ji, S.F.; Liu, Y.W.; Cao, X.; Tian, S.B.; Chen, Y.J.; Niu, Z.Q.; Li, Y.D. Well-Defined Materials for Heterogeneous Catalysis: From Nanoparticles to Isolated Single-Atom Sites. Chem. Rev. 2020, 120, 623–682. [Google Scholar]
- Chung, I.-M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [PubMed] [Green Version]
- Yew, Y.-P.; Shameli, K.; Miyake, M.; Kuwano, N.; Bt Ahmad Khairudin, N.B.; Bt Mohamad, S.E.; Lee, K.-X. Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (Kappaphycusalvarezii) extract. Nanoscale Res. Lett. 2016, 11, 276. [Google Scholar] [CrossRef] [Green Version]
- Kostyukhin, E.M.; Kustov, L.M. Microwave-assisted synthesis of magnetite nanoparticles possessing superior magnetic properties. Mendeleev Commun. 2018, 28, 559–561. [Google Scholar] [CrossRef]
- Mihai, A.D.; Chircov, C.; Grumezescu, A.M.; Holban, A.M. Magnetite Nanoparticles and Essential Oils Systems for Advanced Antibacterial Therapies. Int. J. Mol. Sci. 2020, 21, 7355. [Google Scholar] [CrossRef] [PubMed]
- Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers. Nanomaterials 2020, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- El-Desouky, M.G.; Hassan, N.; Shahat, A.; El-Didamony, A.; El-Bindary, A.A. Synthesis and Characterization of Porous Magnetite Nanosphere Iron Oxide as a Novel Adsorbent of Anionic Dyes Removal from Aqueous Solution. Biointerface Res. Appl. Chem. 2021, 11, 13377–13401. [Google Scholar]
- Wang, N.; Sun, Q.M.; Bai, R.S.; Li, X.; Guo, G.Q.; Yu, J.H. In situ confinement of ultrasmall Pd clusters within nanosized silicate-1 zeolite for highly efficient catalysis of hydrogen generation. J. Am. Chem. Soc. 2016, 138, 7484–7487. [Google Scholar] [CrossRef]
- Silva, V.A.J.; Andrade, P.L.; Silva, M.P.C.; Bustamante, A.D.; De Los Santos Valladares, L.; Albino Aguiar, J. Synthesis and characterization of Fe3O4 nanoparticles coated with fucan polysaccharides. J. Magn. Magn. Mater. 2013, 343, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Dong, W.F.; Sun, H.B. Multifunctional superparamagnetic iron oxide nanoparticles: Design, synthesis and biomedical photonic applications. Nanoscale 2013, 5, 7664–7684. [Google Scholar] [CrossRef] [PubMed]
- Sirivat, A.; Paradee, N. Facile synthesis of gelatin-coated Fe3O4 nanoparticle: Effect of pH in single-step co-precipitation for cancer drug loading. Mater. Des. 2019, 181, 107942. [Google Scholar]
- Lv, Q.; Li, G.; Lu, H.; Cai, W.; Huang, H.; Cheng, C. Preparation of magnetic zeolite γ-Fe2O3/TS-1 with core/shell structure and application in photocatalytic degradation. Microporous Mesoporous Mater. 2015, 203, 202–207. [Google Scholar]
- Loiola, A.R.; Bessa, R.A.; Oliveira, C.P.; Freitas, A.D.L.; Soares, S.A.; Bohn, F.; Pergher, S.B.C. Magnetic zeolite composites: Classification, synthesis routes and technological applications. J. Magn. Magn. Mater. 2022, 560, 169651. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chiang, A.S.T.; Selvin, R.; Thompson, R.W. Rapid Synthesis of MFI Zeolite Nanocrystals. J. Phys. Chem. B 2005, 109, 18804–18814. [Google Scholar] [CrossRef]
- Compeán-Jasso, M.E.; Ruiz, F.; Martínez, J.R.; Herrera-Gómez, A. Magnetic properties of magnetite nanoparticles synthesized by forced hydrolysis. Mater. Lett. 2008, 62, 4248–4250. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.; Li, Z.; Zhu, Y. Synthesis of TS-1 encapsulated Pt (Pt@TS-1) catalyst with hierarchical pores and its enhanced performance in the oxidation of toluene. Microporous Mesoporous Mater. 2023, 350, 112464. [Google Scholar] [CrossRef]
- Nyankson, E.; Adjasoo, A.; Efavi, J.K.; Amedalor, R.; Yaya, Y.; Manu, G.P.; Asare, K.; Amartey, N.A. Characterization and Evaluation of Zeolite A/Fe3O4 Nanocomposite as a Potential Adsorbent for Removal of Organic Molecules from Wastewater. J. Chem. 2019, 2019, 8090756. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhai, Y.; Zhang, X.; Lv, G.; Shen, Y.; Wang, X.; Jiang, T.; Wu, Y. Iron-Containing TS-1 Zeolites with Controllable Mesopores by Desilication and Their Application in Phenol Hydroxylation. Ind. Eng. Chem. Res. 2020, 59, 10289–10297. [Google Scholar] [CrossRef]
- El-Din, T.S.; Elzatahry, A.A.; Aldhayan, D.M.; Al-Enizi, A.M.; Al-Deyab, S.S. Synthesis and characterization of magnetite zeolite nano composite. Int. J. Electrochem. Sci. 2011, 6, 6177–6183. [Google Scholar] [CrossRef]
- Hsu, H.L.; Roselin, L.S.; Savidha, R.; Selvin, R. Enhanced photocatalytic performance of magnetite/TS-1 thin film for phenol degradation. J. Saudi Chem. Soc. 2022, 26, 101538. [Google Scholar] [CrossRef]
- Jamshidi, P.; Shemirani, F. Synthesis of a magnetic WO3 nanocomposite for use in highly selective preconcentration of Pb(II) prior to its quantification by FAAS. Micro-Chim Acta 2018, 185, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Croston, M.; Langston, J.; Sangoi, R.; Santhanam, S.V. Catalytic oxidation of p-toluidine at multiwalled functionalized carbon nanotubes. Int. J. Nanosci. Nanotechnol. 2002, 1, 277–283. [Google Scholar] [CrossRef]
- Daniels, D.G.H.; Naylor, F.T.; Saunders, B.C. Studies in peroxidase action. Part VII. The oxidation of p-toluidine by hydrogen peroxide in the presence of ferrous sulphate. J. Chem. Soc. 1951, 3433–3435. [Google Scholar] [CrossRef]
- Khouw, C.; Dartt, C.B.; Labinger, J.A.; Davis, M.E. Studies on the catalytic oxidation of alkanes and alkenes by titanium silicates. J. Catal. 1994, 149, 195–205. [Google Scholar] [CrossRef]
- Sreeja, V.; Jayaprabha, K.N.; Joy, P.A. Water-dispersible ascorbic-acid-coated magnetite nanoparticles for contrast enhancement in MRI. Appl. Nanosci. 2015, 5, 435–441. [Google Scholar] [CrossRef] [Green Version]








| Sample | Particle Size (nm) | Polyindex |
|---|---|---|
| M | 4.1 | 0.02 |
| TCP-0 | 4.2 | 0.58 |
| SCP-0 | 4 | 0.54 |
| NTS | 39 | 0.05 |
| NS | 36.9 | 0.03 |
| M/NTS | 46.7 | 0.04 |
| M/NS | 43.6 | 0.03 |
| Catalyst (M/NTS) | Conversion of p-Toluidine | Product Distribution | |
|---|---|---|---|
| 4,4′-Dimethylazobenzene | 4,4′-Dimethylazoxybenzene | ||
| Fresh catalyst | 90 | 93 | 7 |
| 1st reuse | 88.7 | 93 | 7 |
| 2nd reuse | 88 | 93 | 7 |
| Catalyst | Conversion of p-Toluidine | Product Distribution | |
|---|---|---|---|
| 4,4′-Dimethylazobenzene | 4,4′-Dimethylazoxybenzene | ||
| M/NTS | 90 | 93 | 7 |
| M/NS | 67.8 | 88 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, K.H.; Alharbi, W.; Alhayyani, S.; Roselin, L.S.; Selvin, R. Enhanced Oxidation of p-Toluidine Using Supported Zeolite Nanoparticles. Molecules 2023, 28, 5737. https://doi.org/10.3390/molecules28155737
Alharbi KH, Alharbi W, Alhayyani S, Roselin LS, Selvin R. Enhanced Oxidation of p-Toluidine Using Supported Zeolite Nanoparticles. Molecules. 2023; 28(15):5737. https://doi.org/10.3390/molecules28155737
Chicago/Turabian StyleAlharbi, Khadijah H., Walaa Alharbi, Sultan Alhayyani, L. Selva Roselin, and Rosilda Selvin. 2023. "Enhanced Oxidation of p-Toluidine Using Supported Zeolite Nanoparticles" Molecules 28, no. 15: 5737. https://doi.org/10.3390/molecules28155737