The Potential Role of Timosaponin-AIII in Cancer Prevention and Treatment
Abstract
:1. Introduction
2. Chemical Structure, Biotransformation, and Structure–Activity Relationship of TSAIII
3. Pharmacokinetic Profiles of TSAIII
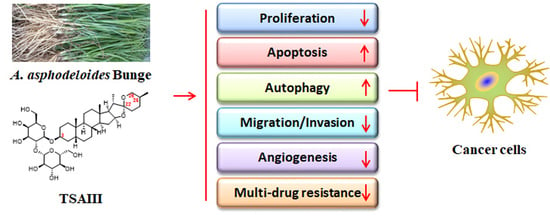
4. The Anticancer Effects of TSAIII
4.1. Proliferation Inhibition, Cell Cycle Arrest, and Apoptosis Induction
4.2. Autophagy Mediation
4.3. Suppression of Migration and Invasion
4.4. Anti-Angiogenesis
4.5. Reverse of Multidrug Resistance
4.6. Anti-Inflammation
4.7. Antioxidant Effects
4.8. Miscellaneous
| Cancer Types | Cell Lines | Concentrations | Key Molecular Targets or Signaling Pathways | Effects | Refs. |
|---|---|---|---|---|---|
| Pancreatic cancer | PANC-1, BxPC-3 | 5, 10, 20 μM | Cell cycle G1 arrest, p-BAD↓, p-mTOR↓, p-p70S6↓, cleaved caspase↑ | Causes cell cycles, inhibits proliferation, and induces apoptosis | [37] |
| AsPC-1 | 5, 20 μM | Cell cycle G1 and G2/M arrest, p-ERK1/2↓, p-STAT3↓, Apoptotic rate↑, p-c-Src kinase↓, Bcl-2↓, MMP-9↓, VEGF-1↓, cyclin D1↑, p21↑ | Causes cell cycles, induces apoptosis, and inhibits proliferation, metastasis, and angiogenesis | [40] | |
| BxPC-3 | 2.5, 5 μM | Mature SREBP-1↓, FASN↓, ACC↓, HMGCR↓, cell cycle G0/G1 arrest, cyclin E1↓, cyclin D1↓, CDK2↓, CDK6↓, p21↑, p27↑, cleaved caspase-3↑, cleaved caspase-9↑, cleaved PARP↑, Bid↑ | Causes cell cycles, induces apoptosis, and inhibits proliferation | [50] | |
| Leukemia | HL60 | 2, 4, 8 μM | Apoptotic rate↑, cleaved caspase-3↑, caspase-8↑, caspase-9↑, and PARP↑, p-JNK1/2↑, p-p38↑ | Induces apoptosis | [38] |
| Jurkat | 2, 8 μM | Proliferation↓, apoptotic rate↑, Bcl-2↓, Bax↑, LC3-II↑, Beclin 1↑, p-PI3K↓, p-AKT↓, p-mTOR↓ | Inhibits proliferation and induces apoptosis and autophagy | [63] | |
| K562/ADM | 1, 2 μM | Intracellular accumulation of ADM↑, P-gp↓, MRP1↓, p-AKT↓ | Reverses drug resistance | [89] | |
| Breast cancer | MDA-MB-231, BT474 | 5 μM | caspase-4↑, Bim↑, REDD1/DDIT4↑, p21CIP↑, stratifin↑, GDF15↑, Myc↓, Id1↓, Id3↓, mTOR↓, eIF2α↑, CHOP↑, LC3-II↑ | Induces apoptosis and autophagy | [20] |
| MDA-MB-231, MCF7 | 10, 15 μM | Cell cycle G2/M arrest, Cdc25C↓, CyclinB1↓, Cdc2↓, Ki67↓, PCNA↓, Bcl-2/Bax ratio↓, caspase-3↑, ATM↑, γ-H2AX↑, p-p38/p38↑ | Causes cell cycles, inhibits proliferation, and induces apoptosis | [39] | |
| MDA-MB-231, MCF7 | 2, 4 μM | Proliferation↓, migration↓, invasion↓, BMI1↓, H2AUb↓, c-Myc↓, miR-200c↑, miR-141↑ | Inhibits proliferation and inhibits migration and invasion | [77] | |
| MDA-MB-231 | 10−8, 10−7, 10−6 M | Invasion↓, COX-2↓, p-ERK↓, p-cMet↓, MMP-9↓, ROS↑ | Inhibits invasion | [78] | |
| Colon cancer | HCT-15 | 5, 10, 20 μM | IC50 = 6.1 μM, cell cycle G0/G1 and G2/M arrest, cyclin A↓, cyclin B1↓, CDK2↓, CDK4↓, proliferating cell nuclear antigen↓, c-Myc↓, Bcl-2↓, Bcl-xL↓, cleaved caspase-3↑, caspase-8↑, caspase-9↑, PARP↑ | Causes cell cycles, inhibits proliferation, and induces apoptosis | [41] |
| HCT116 | 7.5, 10, 12.5 μM | MID1IP1↓, CNOT2↓, c-Myc↓, c-Myc stability↓, caspase-3↑, PARP↑ | Inhibits proliferation and induces apoptosis | [55] | |
| Liver cancer | HepG2 | 6, 9, 12, 15 μM | IC50 = 15.41 μM, apoptotic rate↑, caspase-3↑, caspase-7↑, caspase-8↑, caspase-9↑, Bcl-2↓, Mcl-1↓, cIAP-1↓, cIAP-2↓, XIAP↓, survivin↓, livin↓ | Induces mitochondria-mediated and caspase-dependent apoptosis | [42] |
| HepG2 MHCC97L PLC/PRF/5 Hep3B | 5, 10, 20 μM | Cleaved caspase-3↑, PARP↑, XIAP↓, AMPKα↑, mTOR↓, LC3-II↑, p-S6K↑, p-S6↑ | Induces apoptosis and autophagy | [58] | |
| Nasopharyngeal cancer | CNE-1, HNE-2 | 15 μM TSAIII + 8 Μm PTX | Apoptotic rate↑, Bad↑, RAP1GAP↑, Bcl-2↓, RAP1↓, RasGRP2↓ | Inhibits proliferation and induces apoptosis | [45] |
| Glioma | U87MG | 5, 10 μM | β-catenin↓, cyclin D1↓, Bcl-2↓, PDE5↓, sGCβ↑, cGMP↑, PKG↑, p-VASP-Ser239↑ | Inhibits proliferation and induces apoptosis | [47] |
| GBM8401 and M059K | 5, 10, 15 μM | IC50 = 9.5 and 8.9 μM, respectively. Cleaved caspase-3↑, caspase-9↑, PARP↑, ΔΨm↓, cyto c↑, Mcl-1↓, p62↑, LC3-II↑, LAMP1↑ | Induces autophagy and apoptosis | [60] | |
| Cervical cancer | HeLa | 10 μM | Autophagic flux↑, p62↓, LC3-II↑, p70S6K↓, ULK1↑, mTOR↓ | Induces autophagy | [57] |
| 5, 10, 15, 20 μM | LC3-II↑, ROS↑, SOD↑, CAT↑, ΔΨm↓, cyto c↑, caspase-3↑ | Induces autophagy, apoptosis, and oxidative stress | [59] | ||
| SiHa, HeLa | 2, 4, 6 μM | migration↓, invasion↓, uPA↓, p-p38↓, SOX2↓, OCT4↓, CD49f↓, Nanog↓ | Inhibits migration and invasion | [73] | |
| Lung cancer | A549, H1299 | 20 μM | IC50 = 15.33 and 20.95 μM, respectively. Cleaved caspase-3↑, caspase-8↑, caspase-9↑, PARP↑, Cyto c↑, AIF↑, EndoG↑, Bax↑, LC3-II↑, p-AMPK↑, ERK1/2↓ | Induces autophagy and apoptosis | [61] |
| A549 | 3, 6, 9 μM | Apoptosis↑, migration↓, invasion↓, MMP-2↓, MMP-9↓, p-ERK1/2↓, p-FAK↓, p-Src↓, β-catenin↓ | Induces apoptosis and inhibits migration and invasion | [74] | |
| A549/Taxol, A2780/Taxol | 2, 4, 8 μM | IC50 = 5.12 and 4.64 μM, respectively. Apoptosis↑, Bax↑, Bcl-2↓, PARP↓, PI3K↓, AKT↓, p-AKT↓, mTOR↓, Ras↓, Raf↓, MEK↓, ERK↓, p-ERK↓ | Induces apoptosis and reverses drug resistance | [90] | |
| A549, H1299 | 1, 2, 4 μM | Apoptosis↑, Cell cycle G2/M arrest, migration↓, vimentin↓, Snail-2↓, Snail-1↓, MMP-9↓, E-cadherin↑, ROS↑, MDA↑, iron accumulation↑, HMOX-1↑, FTL↓, GPX4↓, SLC40A1↓, SLC7A11↓ | Induces apoptosis, causes cell cycle arrest, inhibits migration, and induces ferroptosis | [103] | |
| Melanoma | A375-S2 | 2, 4, 6 μM | Cell cycle G1 arrest, cleaved caspase-3↑, LC3-II↑, Beclin 1↑, p-JNK↑, p-ERK↑ | Causes cell cycle arrest and induces autophagy and apoptosis | [62] |
| B16-F10 | 10, 50, 100, 200 nM | migration↓, invasion↓, COX-2↓, PGE2↓, EP2↓, EP4↓, p65↓, IKKα↓, IκBα↓ | Inhibits migration and invasion | [70] | |
| Bone cancer | 143-B, HOS | 2, 4, 6 μM | F-actin↓, migration↓, invasion↓, integrin-αv↓, integrin-β3↓, p-FAK, p-Src↓, TESK1↑, p-cofilin↑ | Inhibits migration and invasion | [67] |
| MG63 | 6 μM TSAIII + 250 μM Rg1 | Apoptotic rate↑, caspase-3↑, migration↓, MMP-2↓, MMP-9↓, p-ERK↓, p-JNK↓, p-p38↓, p-CREB↓, β-catenin↓ | Induce apoptosis and inhibits migration | [79] | |
| MG63 | 3, 6 μM | Migration↓, invasion↓, MMP-2↓, MMP-9↓, p-FAK↓, p-Src↓, p-ERK↓, p-JNK↓, p-p38↓, p-CREB↓, β-catenin↓, cleaved PARP↑ | Inhibits migration and invasion and induces apoptosis | [80] | |
| Renal cancer | 786-O, A-498 | 2, 4, 6 μM | CTSC↓, migration↓, invasion↓, p-PI3K↓, p-AKT↓, miR-129-5p↑ | Inhibits migration and invasion | [71] |
5. Perspectives and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Çetinkaya, M.; Baran, Y. Therapeutic Potential of Luteolin on Cancer. Vaccines 2023, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Sleire, L.; Førde, H.E.; Netland, I.A.; Leiss, L.; Skeie, B.S.; Enger, P. Drug repurposing in cancer. Pharmacol. Res. 2017, 124, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Megyesfalvi, Z.; Gay, C.M.; Popper, H.; Pirker, R.; Ostoros, G.; Heeke, S.; Lang, C.; Hoetzenecker, K.; Schwendenwein, A.; Boettiger, K.; et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA A Cancer J. Clin. 2023. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Luo, D.; Luesch, H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dan, Y.; Yang, D.; Hu, Y.; Zhang, L.; Zhang, C.; Zhu, H.; Cui, Z.; Li, M.; Liu, Y. The genus Anemarrhena Bunge: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2014, 153, 42–60. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Ismail, M.; Khurram, M.; Ullah, I.; Rabbi, F.; Iriti, M. Bioactive Steroids and Saponins of the Genus Trillium. Molecules 2017, 22, 2156. [Google Scholar] [CrossRef] [Green Version]
- Han, F.Y.; Song, X.Y.; Chen, J.J.; Yao, G.D.; Song, S.J. Timosaponin AIII: A novel potential anti-tumor compound from Anemarrhena asphodeloides. Steroids 2018, 140, 125–130. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, W.R.; Shi, W.T.; Zhang, J.; Zhang, K.Y.; Ding, Q.; Chen, X.L.; Tang, J.Y.; Zhou, Z.Y. Pharmacological Activity, Pharmacokinetics, and Toxicity of Timosaponin AIII, a Natural Product Isolated From Anemarrhena asphodeloides Bunge: A Review. Front. Pharmacol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Tang, Y.H.; Sun, Z.L.; Fan, M.S.; Li, Z.X.; Huang, C.G. Anti-diabetic effects of TongGuanWan, a Chinese traditional herbal formula, in C57BL/KsJ-db/db mice. Planta Med. 2012, 78, 18–23. [Google Scholar] [CrossRef]
- Tang, Z.; Li, G.; Yang, J.; Duan, J.; Qian, D.; Guo, J.; Zhu, Z.; Song, Z. Anemarrhena asphodeloides Non-Steroidal Saponin Components Alter the Pharmacokinetic Profile of Its Steroidal Saponins in Rat. Molecules 2015, 20, 11777–11792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Liu, Z.; Wang, Q.; Chai, Y.; Xia, P. Pharmacokinetic comparison of seven major bioactive components in normal and depression model rats after oral administration of Baihe Zhimu decoction by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018, 148, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Li, T.; Fong, C.M.; Chen, X.; Chen, X.J.; Wang, Y.T.; Huang, M.Q.; Lu, J.J. Saponins from Chinese Medicines as Anticancer Agents. Molecules 2016, 21, 1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tundis, R.; Bonesi, M.; Menichini, F.; Loizzo, M.R. Recent Knowledge on Medicinal Plants as Source of Cholinesterase Inhibitors for the Treatment of Dementia. Mini Rev. Med. Chem. 2016, 16, 605–618. [Google Scholar] [CrossRef]
- Wang, N.; Xu, P.; Wang, X.; Yao, W.; Wang, B.; Wu, Y.; Shou, D. Timosaponin AIII attenuates inflammatory injury in AGEs-induced osteoblast and alloxan-induced diabetic osteoporosis zebrafish by modulating the RAGE/MAPK signaling pathways. Phytomedicine 2020, 75, 153247. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Jeong, J.J.; Kang, G.D.; Kim, K.A.; Choi, H.S.; Kim, D.H. Timosaponin AIII and its metabolite sarsasapogenin ameliorate colitis in mice by inhibiting NF-κB and MAPK activation and restoring Th17/Treg cell balance. Int. Immunopharmacol. 2015, 25, 493–503. [Google Scholar] [CrossRef]
- Lu, L.; Liu, Y.; Ding, Y.; Hou, J.; Zhang, Y.; Xue, H.; Zhang, T. Preparation of highly purified timosaponin AIII from rhizoma anemarrhenae through an enzymatic method combined with preparative liquid chromatography. Nat. Prod. Res. 2016, 30, 2364–2367. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, X.; Pei, H.; Chen, M.C.; Sun, Z.L.; Xue, Y.R.; Tian, X.T.; Huang, C.G. Metabolism, pharmacokinetics, and hepatic disposition of xanthones and saponins on Zhimu treatments for exploratively interpreting the discrepancy between the herbal safety and timosaponin A3-induced hepatotoxicity. Acta Pharmacol. Sin. 2018, 39, 1923–1934. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent Advances in Biotransformation of Saponins. Molecules 2019, 24, 2365. [Google Scholar] [CrossRef] [Green Version]
- King, F.W.; Fong, S.; Griffin, C.; Shoemaker, M.; Staub, R.; Zhang, Y.L.; Cohen, I.; Shtivelman, E. Timosaponin AIII is preferentially cytotoxic to tumor cells through inhibition of mTOR and induction of ER stress. PLoS ONE 2009, 4, e7283. [Google Scholar] [CrossRef]
- Sy, L.K.; Lok, C.N.; Wang, J.Y.; Liu, Y.; Cheng, L.; Wan, P.K.; Leung, C.T.; Cao, B.; Kwong, W.L.; Chang, R.C.; et al. Identification of “sarsasapogenin-aglyconed” timosaponins as novel Aβ-lowering modulators of amyloid precursor protein processing. Chem. Sci. 2016, 7, 3206–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Z.; Feng, B.; Huang, H.Z.; Kang, L.P.; Cong, Y.; Zhou, W.B.; Zou, P.; Cong, Y.W.; Song, X.B.; Ma, B.P. Glucosylation of steroidal saponins by cyclodextrin glucanotransferase. Planta Med. 2010, 76, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhao, X.C.; Wang, S.J.; Gao, P.Y.; Li, L.Z.; Ikejima, T.; Song, S.J. Synthesis and biological evaluation of novel sarsasapogenin derivatives as potential anti-tumor agents. Steroids 2015, 93, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, D.; Wang, Z.; Yao, G.; Li, X.; Gao, P.; Li, L.; Zhang, Y.; Wang, S.; Song, S. Synthesis of new sarsasapogenin derivatives with cytotoxicity and apoptosis-inducing activities in human breast cancer MCF-7 cells. Eur. J. Med. Chem. 2017, 127, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, X.; Ding, X.; Chen, X.; Lv, L.; Li, Y.; Chai, Y. Comparative pharmacokinetics of timosaponin B-II and timosaponin A-III after oral administration of Zhimu-Baihe herb-pair, Zhimu extract, free timosaponin B-II and free timosaponin A-III to rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 926, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pu, Y.; Zhang, T.; Ding, Y.; Wang, B.; Cai, Z. Rapid and Sensitive Determination of Timosaponin AIII in Rat Plasma by LC-MS/MS and Its Pharmacokinetic Application. Int. J. Mol. Sci. 2013, 14, 3656–3670. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Liu, Z.; Hu, J.; Lai, X.; Xia, P. Quantitative determination of sarsasapogenin in rat plasma using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1022, 213–219. [Google Scholar] [CrossRef]
- Wang, H.Q.; Lan, F.; Zhang, Y.H.; Xia, J.E.; Gong, X.M.; Liu, M. Identification and pharmacokinetics of saponins in Rhizoma anemarrhenae after oral administration to rats by HPLC-Q-TOF/MS and HPLC-MS/MS. Acta Pharm. 2021, 71, 567–585. [Google Scholar] [CrossRef]
- Li, G.; Tang, Z.; Yang, J.; Duan, J.; Qian, D.; Guo, J.; Zhu, Z.; Liu, H. Simultaneous determination of five components in rat plasma by UPLC-MS/MS and its application to a comparative pharmacokinetic study in Baihe Zhimu Tang and Zhimu extract. Molecules 2015, 20, 6700–6714. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Wu, B.; Fan, M.; Wang, J.; Huang, J.; Huang, C. High-performance liquid chromatography-electrospray ionization tandem mass spectrometry for metabolism study of timosaponin AIII. J. Chromatogr. Sci. 2014, 52, 418–422. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, L.; Peng, Y.; Liu, B.; Lin, D.; Li, L.; Song, S. Metabolites characterization of timosaponin AIII in vivo and in vitro by using liquid chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 997, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Y.; Pei, L.; Gao, H. Comparison of the pharmacokinetics of timosaponin AIII, timosaponin BIII, and mangiferin extracted from crude and salt-processed Anemarrhenae Rhizoma by UPLC-MS/MS. RSC Adv. 2023, 13, 11919–11928. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ding, Y.; Zhang, Y.; Ho, R.J.; Zhao, Y.; Zhang, T.; Guo, C. Antibody-modified liposomes for tumor-targeting delivery of timosaponin AIII. Int. J. Nanomed. 2018, 13, 1927–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; He, Y.; Zhang, M.; Lu, Z.; Zhang, T.; Wang, B. GPP-TSAIII nanocomposite hydrogel-based photothermal ablation facilitates melanoma therapy. Expert Opin. Drug Deliv. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ford, H.L.; Pardee, A.B. Cancer and the cell cycle. J. Cell Biochem. 1999, 32, 166–172. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Hengartner, M.O. Programmed cell death: Alive and well in the new millennium. Trends Cell Biol. 2001, 11, 526–534. [Google Scholar] [CrossRef]
- MarElia, C.B.; Sharp, A.E.; Shemwell, T.A.; Clare Zhang, Y.; Burkhardt, B.R. Anemarrhena asphodeloides Bunge and its constituent timosaponin-AIII induce cell cycle arrest and apoptosis in pancreatic cancer cells. FEBS Open Bio 2018, 8, 1155–1166. [Google Scholar] [CrossRef]
- Huang, H.L.; Chiang, W.L.; Hsiao, P.C.; Chien, M.H.; Chen, H.Y.; Weng, W.C.; Hsieh, M.J.; Yang, S.F. Timosaponin AIII mediates caspase activation and induces apoptosis through JNK1/2 pathway in human promyelocytic leukemia cells. Tumour Biol. 2015, 36, 3489–3497. [Google Scholar] [CrossRef]
- Zhang, M.; Qu, J.; Gao, Z.; Qi, Q.; Yin, H.; Zhu, L.; Wu, Y.; Liu, W.; Yang, J.; Huang, X. Timosaponin AIII Induces G2/M Arrest and Apoptosis in Breast Cancer by Activating the ATM/Chk2 and p38 MAPK Signaling Pathways. Front. Pharmacol. 2020, 11, 601468. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.H.; Lee, I.S.; Park, J.Y.; Na, Y.C.; Chung, W.S.; Jang, H.J. Apoptosis and G2/M cell cycle arrest induced by a timosaponin A3 from Anemarrhena asphodeloides Bunge on AsPC-1 pancreatic cancer cells. Phytomedicine 2019, 56, 48–56. [Google Scholar] [CrossRef]
- Kang, Y.J.; Chung, H.J.; Nam, J.W.; Park, H.J.; Seo, E.K.; Kim, Y.S.; Lee, D.; Lee, S.K. Cytotoxic and antineoplastic activity of timosaponin A-III for human colon cancer cells. J. Nat. Prod. 2011, 74, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Nho, K.J.; Chun, J.M.; Kim, H.K. Induction of mitochondria-dependent apoptosis in HepG2 human hepatocellular carcinoma cells by timosaponin A-III. Environ. Toxicol. Pharmacol. 2016, 45, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Looi, C.K.; Hii, L.W.; Ngai, S.C.; Leong, C.O.; Mai, C.W. The Role of Ras-Associated Protein 1 (Rap1) in Cancer: Bad Actor or Good Player? Biomedicines 2020, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, W.; Zhou, T.; Zhao, F.; Yang, L. Timosaponin AIII Suppresses RAP1 Signaling Pathway to Enhance the Inhibitory Effect of Paclitaxel on Nasopharyngeal Carcinoma. Comput. Math. Methods Med. 2022, 2022, 6756676. [Google Scholar] [CrossRef]
- Lou, W.; Ding, B.; Xu, L.; Fan, W. Construction of Potential Glioblastoma Multiforme-Related miRNA-mRNA Regulatory Network. Front. Mol. Neurosci. 2019, 12, 66. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.F.; Pan, H.J.; Abudurezeke, N.; Yuan, C.L.; Yuan, Y.L.; Zhao, S.D.; Zhang, D.D.; Huang, S. Functional Axis of PDE5/cGMP Mediates Timosaponin-AIII-Elicited Growth Suppression of Glioblastoma U87MG Cells. Molecules 2023, 28, 3795. [Google Scholar] [CrossRef]
- Shao, W.; Espenshade, P.J. Expanding roles for SREBP in metabolism. Cell Metab. 2012, 16, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Ru, P.; Geng, F.; Liu, J.; Yoo, J.Y.; Wu, X.; Cheng, X.; Euthine, V.; Hu, P.; Guo, J.Y.; et al. Glucose-Mediated N-glycosylation of SCAP Is Essential for SREBP-1 Activation and Tumor Growth. Cancer Cell 2015, 28, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Jee, W.; An, E.J.; Ko, H.M.; Jung, J.H.; Na, Y.C.; Jang, H.J. Timosaponin A3 Inhibits Palmitate and Stearate through Suppression of SREBP-1 in Pancreatic Cancer. Pharmaceutics 2022, 14, 945. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Jiang, M.; Lu, L.; Ding, Y.; Ma, N.; Zhao, Y.; Xuchen, S.; Zhang, N. Novel Timosaponin AIII-Based Multifunctional Liposomal Delivery System for Synergistic Therapy Against Hepatocellular Carcinoma Cancer. Int. J. Nanomed. 2021, 16, 5531–5550. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, D.; Ko, H.M.; Jang, H.J. Inhibition of CNOT2 Induces Apoptosis via MID1IP1 in Colorectal Cancer Cells by Activating p53. Biomolecules 2021, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Sim, D.Y.; Lee, H.M.; Lee, H.J.; Kim, S.H. Hypolipogenic Effect of Shikimic Acid Via Inhibition of MID1IP1 and Phosphorylation of AMPK/ACC. Int. J. Mol. Sci. 2019, 20, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Lee, H.J.; Kim, J.H.; Sim, D.Y.; Im, E.; Kim, S.; Chang, S.; Kim, S.H. Colocalization of MID1IP1 and c-Myc is Critically Involved in Liver Cancer Growth via Regulation of Ribosomal Protein L5 and L11 and CNOT2. Cells 2020, 9, 985. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.M.; Jee, W.; Park, D.I.; Kim, K.I.; Jung, J.H.; Jang, H.J. The Antitumor Effect of Timosaponin A3 through c-Myc Inhibition in Colorectal Cancer Cells and Combined Treatment Effect with 5-FU or Doxorubicin. Int. J. Mol. Sci. 2022, 23, 11900. [Google Scholar] [CrossRef]
- Knaevelsrud, H.; Simonsen, A. Fighting disease by selective autophagy of aggregate-prone proteins. FEBS Lett. 2010, 584, 2635–2645. [Google Scholar] [CrossRef] [Green Version]
- Lok, C.N.; Sy, L.K.; Liu, F.; Che, C.M. Activation of autophagy of aggregation-prone ubiquitinated proteins by timosaponin A-III. J. Biol. Chem. 2011, 286, 31684–31696. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Feng, Y.; Zhu, M.; Siu, F.M.; Ng, K.M.; Che, C.M. A novel mechanism of XIAP degradation induced by timosaponin AIII in hepatocellular carcinoma. Biochim. Biophys. Acta 2013, 1833, 2890–2899. [Google Scholar] [CrossRef] [Green Version]
- Sy, L.K.; Yan, S.C.; Lok, C.N.; Man, R.Y.; Che, C.M. Timosaponin A-III induces autophagy preceding mitochondria-mediated apoptosis in HeLa cancer cells. Cancer Res. 2008, 68, 10229–10237. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; Tsai, J.P.; Lee, H.L.; Chen, Y.J.; Chen, Y.S.; Hsieh, Y.H.; Chen, J.C. Blockage of Autophagy Increases Timosaponin AIII-Induced Apoptosis of Glioma Cells In Vitro and In Vivo. Cells 2022, 12, 168. [Google Scholar] [CrossRef]
- Liu, J.; Deng, X.; Sun, X.; Dong, J.; Huang, J. Inhibition of autophagy enhances timosaponin AIII-induced lung cancer cell apoptosis and anti-tumor effect in vitro and in vivo. Life Sci. 2020, 257, 118040. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Lou, L.L.; Song, S.J.; Yao, G.D.; Ge, M.Y.; Hayashi, T.; Tashiro, S.I.; Onodera, S.; Ikejima, T. Timosaponin AIII induces apoptosis and autophagy in human melanoma A375-S2 cells. Arch. Pharm. Res. 2017, 40, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, R.; Fan, W.W.; Zheng, X.C.; Li, A.M.; Wang, W.D. Timosaponin A-III induces autophagy of T-cell acute lymphoblastic leukemia Jurkat cells via inhibition of the PI3K/Akt/mTOR pathway. Oncol. Rep. 2019, 41, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Panda, C.K.; Nandi, S.; Mukhopadhyay, A. An insight into metastasis: Random or evolving paradigms? Pathol. Res. Pract. 2018, 214, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Gilcrease, M.Z. Integrin signaling in epithelial cells. Cancer Lett. 2007, 247, 1–25. [Google Scholar] [CrossRef]
- Wen, L.; Yan, W.; Zhu, L.; Tang, C.; Wang, G. The role of blood flow in vessel remodeling and its regulatory mechanism during developmental angiogenesis. Cell. Mol. Life Sci. CMLS 2023, 80, 162. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Hsu, W.H.; Yang, S.F.; Liu, C.J.; Lu, K.H.; Wang, P.H.; Lin, R.C. Potential Antimetastatic Effect of Timosaponin AIII against Human Osteosarcoma Cells through Regulating the Integrin/FAK/Cofilin Axis. Pharmaceuticals 2021, 14, 260. [Google Scholar] [CrossRef]
- Riedl, K.; Krysan, K.; Põld, M.; Dalwadi, H.; Heuze-Vourc’h, N.; Dohadwala, M.; Liu, M.; Cui, X.; Figlin, R.; Mao, J.T.; et al. Multifaceted roles of cyclooxygenase-2 in lung cancer. Drug Resist. Updates 2004, 7, 169–184. [Google Scholar] [CrossRef]
- Majumder, M.; Xin, X.; Liu, L.; Girish, G.V.; Lala, P.K. Prostaglandin E2 receptor EP4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci. 2014, 105, 1142–1151. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.M.; Im, A.R.; Kim, S.H.; Hyun, J.W.; Chae, S. Timosaponin AIII inhibits melanoma cell migration by suppressing COX-2 and in vivo tumor metastasis. Cancer Sci. 2016, 107, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Chiang, K.C.; Lai, C.Y.; Chiou, H.L.; Lin, C.L.; Chen, Y.S.; Kao, S.H.; Hsieh, Y.H. Timosaponin AIII inhibits metastasis of renal carcinoma cells through suppressing cathepsin C expression by AKT/miR-129-5p axis. J. Cell Physiol. 2019, 234, 13332–13341. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.H.; Lee, C.H.; Chiou, H.L.; Yang, S.F.; Lin, C.L.; Hung, C.H.; Tsai, J.P.; Hsieh, Y.H. Knockdown of Pentraxin 3 suppresses tumorigenicity and metastasis of human cervical cancer cells. Sci. Rep. 2016, 6, 29385. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.J.; Liu, C.J.; Ying, T.H.; Wu, P.J.; Wang, J.W.; Ting, Y.H.; Hsieh, Y.H.; Wang, S.C. Timosaponin AIII Inhibits Migration and Invasion Abilities in Human Cervical Cancer Cells through Inactivation of p38 MAPK-Mediated uPA Expression In Vitro and In Vivo. Cancers 2022, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Lee, J.; Lee, Y.J.; Yun, J.M.; Son, Y.J.; Cho, J.Y.; Ryou, C.; Lee, S.Y. Timosaponin AIII inhibits migration and invasion of A549 human non-small-cell lung cancer cells via attenuations of MMP-2 and MMP-9 by inhibitions of ERK1/2, Src/FAK and β-catenin signaling pathways. Bioorg. Med. Chem. Lett. 2016, 26, 3963–3967. [Google Scholar] [CrossRef]
- Abdouh, M.; Facchino, S.; Chatoo, W.; Balasingam, V.; Ferreira, J.; Bernier, G. BMI1 sustains human glioblastoma multiforme stem cell renewal. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 8884–8896. [Google Scholar] [CrossRef]
- Song, L.B.; Zeng, M.S.; Liao, W.T.; Zhang, L.; Mo, H.Y.; Liu, W.L.; Shao, J.Y.; Wu, Q.L.; Li, M.Z.; Xia, Y.F.; et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006, 66, 6225–6232. [Google Scholar] [CrossRef] [Green Version]
- Gergely, J.E.; Dorsey, A.E.; Dimri, G.P.; Dimri, M. Timosaponin A-III inhibits oncogenic phenotype via regulation of PcG protein BMI1 in breast cancer cells. Mol. Carcinog. 2018, 57, 831–841. [Google Scholar] [CrossRef]
- Tsai, C.H.; Yang, C.W.; Wang, J.Y.; Tsai, Y.F.; Tseng, L.M.; King, K.L.; Chen, W.S.; Chiu, J.H.; Shyr, Y.M. Timosaponin AIII Suppresses Hepatocyte Growth Factor-Induced Invasive Activity through Sustained ERK Activation in Breast Cancer MDA-MB-231 Cells. Evid.-Based Complement. Altern. Med. eCAM 2013, 2013, 421051. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y. Ginsenoside Rg1 Drives Stimulations of Timosaponin AIII-Induced Anticancer Effects in Human Osteosarcoma Cells. Evid.-Based Complement. Altern. Med. eCAM 2020, 2020, 8980124. [Google Scholar] [CrossRef]
- Jung, O.; Lee, S.Y. Synergistic anticancer effects of timosaponin AIII and ginsenosides in MG63 human osteosarcoma cells. J. Ginseng Res. 2019, 43, 488–495. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, S.; Fan, D.; Guo, X.; Ma, S. The role of vascular endothelial cells in tumor metastasis. Acta Histochem. 2023, 125, 152070. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Zhao, W.R.; Xiao, Y.; Zhou, X.M.; Huang, C.; Shi, W.T.; Zhang, J.; Ye, Q.; Chen, X.L.; Tang, J.Y. Antiangiogenesis effect of timosaponin AIII on HUVECs in vitro and zebrafish embryos in vivo. Acta Pharmacol. Sin. 2020, 41, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kiran Ammu, V.V.V.; Garikapati, K.K.; Krishnamurthy, P.T.; Chintamaneni, P.K.; Pindiprolu, S. Possible role of PPAR-γ and COX-2 receptor modulators in the treatment of Non-Small Cell lung carcinoma. Med. Hypotheses 2019, 124, 98–100. [Google Scholar] [CrossRef]
- Amano, H.; Nakamura, M.; Ito, Y.; Kakutani, H.; Eshima, K.; Kitasato, H.; Narumiya, S.; Majima, M. Thromboxane A synthase enhances blood flow recovery from hindlimb ischemia. J. Surg. Res. 2016, 204, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Cong, Y.; Wang, L.; Peng, R.; Zhao, Y.; Bai, F.; Yang, C.; Liu, X.; Wang, D.; Ma, B.; Cong, Y. Timosaponin AIII induces antiplatelet and antithrombotic activity via Gq-mediated signaling by the thromboxane A2 receptor. Sci. Rep. 2016, 6, 38757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Lett. 2014, 347, 159–166. [Google Scholar] [CrossRef]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; Feo, V.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; He, Y.; Fang, J.; Wang, H.; Chao, L.; Zhao, L.; Hong, Z.; Chai, Y. In Vitro Evaluation of the Interaction of Seven Biologically Active Components in Anemarrhenae rhizoma with P-gp. Molecules 2022, 27, 8556. [Google Scholar] [CrossRef]
- Chen, J.R.; Jia, X.H.; Wang, H.; Yi, Y.J.; Wang, J.Y.; Li, Y.J. Timosaponin A-III reverses multi-drug resistance in human chronic myelogenous leukemia K562/ADM cells via downregulation of MDR1 and MRP1 expression by inhibiting PI3K/Akt signaling pathway. Int. J. Oncol. 2016, 48, 2063–2070. [Google Scholar] [CrossRef] [Green Version]
- Song, X.Y.; Han, F.Y.; Chen, J.J.; Wang, W.; Zhang, Y.; Yao, G.D.; Song, S.J. Timosaponin AIII, a steroidal saponin, exhibits anti-tumor effect on taxol-resistant cells in vitro and in vivo. Steroids 2019, 146, 57–64. [Google Scholar] [CrossRef]
- Xie, L.; Lee, D.Y.; Shang, Y.; Cao, X.; Wang, S.; Liao, J.; Zhang, T.; Dai, R. Characterization of spirostanol glycosides and furostanol glycosides from anemarrhenae rhizoma as dual targeted inhibitors of 5-lipoxygenase and Cyclooxygenase-2 by employing a combination of affinity ultrafiltration and HPLC/MS. Phytomedicine 2020, 77, 153284. [Google Scholar] [CrossRef] [PubMed]
- Huai, J.; Zhao, X.; Wang, S.; Xie, L.; Li, Y.; Zhang, T.; Cheng, C.; Dai, R. Characterization and screening of cyclooxygenase-2 inhibitors from Zi-shen pill by affinity ultrafiltration-ultra performance liquid chromatography mass spectrometry. J. Ethnopharmacol. 2019, 241, 111900. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huai, J.; Shang, Y.; Xie, L.; Cao, X.; Liao, J.; Zhang, T.; Dai, R. Screening for natural inhibitors of 5-lipoxygenase from Zi-shen pill extract by affinity ultrafiltration coupled with ultra performance liquid chromatography-mass spectrometry. J. Ethnopharmacol. 2020, 254, 112733. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhao, Y.; Zhang, X.; Yang, L.; Zou, X. NLRC3 Participates in Inhibiting the Pulmonary Inflammatory Response of Sepsis-Induced Acute Lung Injury. Immunol. Investig. 2023, 52, 567–582. [Google Scholar] [CrossRef]
- Ji, K.Y.; Kim, K.M.; Kim, Y.H.; Im, A.R.; Lee, J.Y.; Park, B.; Na, M.; Chae, S. The enhancing immune response and anti-inflammatory effects of Anemarrhena asphodeloides extract in RAW 264.7 cells. Phytomedicine 2019, 59, 152789. [Google Scholar] [CrossRef]
- Lee, B.; Jung, K.; Kim, D.H. Timosaponin AIII, a saponin isolated from Anemarrhena asphodeloides, ameliorates learning and memory deficits in mice. Pharmacol. Biochem. Behav. 2009, 93, 121–127. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Cui, S.C.; Zheng, T.N.; Ma, H.J.; Xie, Z.F.; Jiang, H.W.; Li, Y.F.; Zhu, K.X.; Huang, C.G.; Li, J.; et al. Sarsasapogenin improves adipose tissue inflammation and ameliorates insulin resistance in high-fat diet-fed C57BL/6J mice. Acta Pharmacol. Sin. 2021, 42, 272–281. [Google Scholar] [CrossRef]
- Park, B.K.; So, K.S.; Ko, H.J.; Kim, H.J.; Kwon, K.S.; Kwon, Y.S.; Son, K.H.; Kwon, S.Y.; Kim, H.P. Therapeutic Potential of the Rhizomes of Anemarrhena asphodeloides and Timosaponin A-III in an Animal Model of Lipopolysaccharide-Induced Lung Inflammation. Biomol. Ther. 2018, 26, 553–559. [Google Scholar] [CrossRef]
- Kim, K.M.; Im, A.R.; Park, S.K.; Shin, H.S.; Chae, S.W. Protective Effects of Timosaponin AIII against UVB-Radiation Induced Inflammation and DNA Injury in Human Epidermal Keratinocytes. Biol. Pharm. Bull. 2019, 42, 1524–1531. [Google Scholar] [CrossRef] [Green Version]
- Im, A.R.; Seo, Y.K.; Cho, S.H.; O, K.H.; Kim, K.M.; Chae, S. Clinical evaluation of the safety and efficacy of a timosaponin A-III-based antiwrinkle agent against skin aging. J. Cosmet. Dermatol. 2020, 19, 423–436. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Feng, M.; Xing, J.; Zhou, X. Timosaponin alleviates oxidative stress in rats with high fat diet-induced obesity via activating Nrf2/HO-1 and inhibiting the NF-κB pathway. Eur. J. Pharmacol. 2021, 909, 174377. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.T.; Qi, X.M.; Sheng, J.J.; Ma, L.L.; Ni, X.; Ren, J.; Huang, C.G.; Pan, G.Y. Timosaponin A3 induces hepatotoxicity in rats through inducing oxidative stress and down-regulating bile acid transporters. Acta Pharmacol. Sin. 2014, 35, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yu, T.; Zhu, R.; Lu, J.; Ouyang, X.; Zhang, Z.; Chen, Q.; Li, J.; Cui, J.; Jiang, F.; et al. Timosaponin AIII promotes non-small-cell lung cancer ferroptosis through targeting and facilitating HSP90 mediated GPX4 ubiquitination and degradation. Int. J. Biol. Sci. 2023, 19, 1471–1489. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, C.; Liu, Y.; Zhang, S.; Gao, X. Baihe Zhimu formula attenuates the efficacy of tamoxifen against breast cancer in mice through modulation of CYP450 enzymes. BMC Complement. Altern. Med. 2019, 19, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.; Trinh, H.T.; Jung, K.; Han, S.J.; Kim, D.H. Inhibitory effects of steroidal timosaponins isolated from the rhizomes of Anemarrhena asphodeloides against passive cutaneous anaphylaxis reaction and pruritus. Immunopharmacol. Immunotoxicol. 2010, 32, 357–363. [Google Scholar] [CrossRef]
- Xie, H.F.; Li, W.; Pan, H.; Xie, Q.; Hu, Y. Preparation Method of Timosaponin AIII. Chinese Patent CN114085883A, 25 February 2022. [Google Scholar]
- Yuan, F.Y.; Li, X.Y.; Gao, T.; Liu, Z.; Liu, W.; Zhou, D.; Yang, K.; Guo, R.; Li, C.; Tian, Y. Application of Timosaponin AIII in Preparation of Medicine for Inhibiting Haemophilus Parasuis. Chinese Patent CN115317499A, 11 November 2022. [Google Scholar]
- Ding, Y.; Zhao, W.J.; Zhang, T.; Lu, L.; Jiang, M.; Zhang, L.; Zhang, S. Medical Application of Timosaponin Enzymatic Hydrolysate and Main Component Timosaponin AIII (TAIII) Thereof. Chinese Patent CN112168833A, 25 January 2021. [Google Scholar]
- Tao, L.; Duan, G.C.; Gu, Y.; Liu, Y.; Wang, W.; Li, G. Application of Timosaponin A-III in Preparation of Anti-Human Rhabdomyosarcoma Drug. Chinese Patent CN107412245A, 1 December 2017. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Cao, Y.; Guo, X.; Chen, Z. The Potential Role of Timosaponin-AIII in Cancer Prevention and Treatment. Molecules 2023, 28, 5500. https://doi.org/10.3390/molecules28145500
Liu Z, Cao Y, Guo X, Chen Z. The Potential Role of Timosaponin-AIII in Cancer Prevention and Treatment. Molecules. 2023; 28(14):5500. https://doi.org/10.3390/molecules28145500
Chicago/Turabian StyleLiu, Zhaowen, Yifan Cao, Xiaohua Guo, and Zhixi Chen. 2023. "The Potential Role of Timosaponin-AIII in Cancer Prevention and Treatment" Molecules 28, no. 14: 5500. https://doi.org/10.3390/molecules28145500