Sweet Wine Production from the Side-Stream of Industrial Corinthian Currant Processing: Product Quality, Antioxidant Capacity, and Volatilome
Abstract
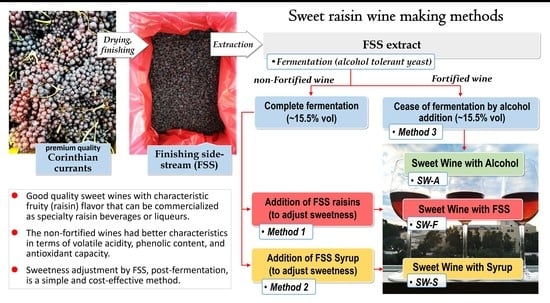
:1. Introduction
2. Results
2.1. Composition of the Sweet Wines Made from FSS
2.2. Volatilome of the Sweet Wines
2.3. Sensory Properties of the Sweet Wines
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Raw Materials, Yeast, and Media
4.3. Preparation of FSS Extracts (Musts)
4.4. Preparation of FSS Syrup
4.5. Sweet Wine Making from FSS
4.5.1. Production of Sweet Wine with Addition of FSS to Adjust Sweetness (SW-F Wine)
4.5.2. Production of Sweet Wine with Addition of FSS Syrup to Adjust Sweetness (SW-S Wine)
4.5.3. Production of Sweet Wine with Potable Alcohol Addition (SW-A Wine)
4.6. Analytical Methods
4.6.1. Determination of Acidity
4.6.2. Determination of Ethanol and Methanol
4.6.3. HPLC Analysis of Sugars and Organic Acids
4.6.4. Determination of TPC, AC, and PPC
4.6.5. Sulfite Analysis
4.6.6. Volatile Profile
4.6.7. Sensory Evaluation
4.6.8. Statistical Analysis and Software
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vantarakis, G.C.; Abeliotis, K.; Karathanos, V.T. Environmental impact assessment of protected designation of origin (PDO) foods: The case of Vostizza Corinthian currants in Greece. Euro-Mediterr. J. Environ. Integr. 2022, 7, 131–140. [Google Scholar] [CrossRef]
- Plioni, I.; Bekatorou, A.; Mallouchos, A.; Kandylis, P.; Chiou, A.; Dede, V.; Styliara, P. Corinthian currants finishing side-stream: Chemical characterization, volatilome, and valorisation through wine and baker’s yeast production-technoeconomic evaluation. Food Chem. 2021, 342, 128161. [Google Scholar] [CrossRef] [PubMed]
- Plioni, I.; Bekatorou, A.; Terpou, A.; Mallouchos, A.; Plessas, S.; Koutinas, A.A.; Katechaki, E. Vinegar production from Corinthian currants finishing side-stream: Development and comparison of methods based on immobilized acetic acid bacteria. Foods 2021, 10, 3133. [Google Scholar] [CrossRef] [PubMed]
- Plioni, I.; Panitsa, A.; Mallouchos, A.; Terpou, A.; Tsogka, I.; Adamopoulou, V.; Bekatorou, A. Production of syrups from Corinthian currant industrial finishing side-stream: Quality evaluation and volatilome. Sustainability 2023, 15, 495. [Google Scholar] [CrossRef]
- Panagopoulou, E.A.; Chiou, A.; Nikolidaki, E.K.; Christea, M.; Karathanos, V.T. Corinthian raisins (Vitis vinifera L., var. Apyrena) antioxidant and sugar content as affected by the drying process: A 3-year study. J. Sci. Food Agric. 2019, 99, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, E.A.; Chiou, A.; Karathanos, V.T. Water-soluble vitamin content of sun-dried Corinthian raisins (Vitis vinifera L., var. Apyrena). J. Sci. Food Agric. 2019, 99, 5327–5333. [Google Scholar] [CrossRef] [PubMed]
- Nikolidaki, E.K.; Chiou, A.; Christea, M.; Gkegka, A.P.; Karvelas, M.; Karathanos, V.T. Sun dried Corinthian currant (Vitis vinifera L., var. Apyrena) simple sugar profile and macronutrient characterization. Food Chem. 2017, 221, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Bekatorou, A.; Plioni, I.; Sparou, K.; Maroutsiou, R.; Tsafrakidou, P.; Petsi, T.; Kordouli, E. Bacterial cellulose production using the Corinthian currant finishing side-stream and cheese whey: Process optimization and textural characterization. Foods 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Rural Development and Food. Hellenic Republic. Strategic Targets for Agricultural Development and Restructuring of the Countryside. Available online: http://www.minagric.gr/index.php/el/the-ministry-2/grafeiotypou/deltiatypou/12801-dt070422 (accessed on 9 May 2023). (In Greek)
- Imtiyaz, H.; Soni, P.; Yukongdi, V. Role of sensory appeal, nutritional quality, safety, and health determinants on convenience food choice in an academic environment. Foods 2021, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Dipalmo, T.; Rizzello, C.G.; Corbo, F.; Crupi, P. Emerging technology to develop novel red winemaking practices: An overview. Innov. Food Sci. Emerg. Technol. 2016, 38, 41–56. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science. Principles and Applications, 3rd ed.; Academic Press: San Diego, CA, USA, 2008; pp. 520–576, 344, 419, 663. [Google Scholar]
- García-Martínez, T.; De Lerma, N.L.; Moreno, J.; Peinado, R.A.; Millán, M.C.; Mauricio, J.C. Sweet wine production by two osmotolerant Saccharomyces cerevisiae strains. J. Food Sci. 2013, 78, M874–M879. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, T.; Moreno, J.; Mauricio, J.C.; Peinado, R. Natural sweet wine production by repeated use of yeast cells immobilized on Penicillium chrysogenum. LWT 2015, 61, 503–509. [Google Scholar] [CrossRef]
- Ruiz-Bejarano, M.J.; Durán-Guerrero, E.; Castro, R.; Barroso, C.G.; Rodríguez-Dodero, M.C. Use of sensory analysis to investigate the influence of climate chambers and other process variables in the production of sweet wines. Foods 2020, 9, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croce, R.; Malegori, C.; Oliveri, P.; Medici, I.; Cavaglioni, A.; Rossi, C. Prediction of quality parameters in straw wine by means of FT-IR spectroscopy combined with multivariate data processing. Food Chem. 2020, 305, 125512. [Google Scholar] [CrossRef] [PubMed]
- International Code of Oenological Practices—2019 Issue. International Organisation of Vine and Wine (OIV). Available online: https://www.oiv.int/public/medias/7713/en-oiv-code-2021.pdf (accessed on 9 May 2023).
- Avizcuri-Inac, J.M.; Gonzalez-Hernandez, M.; Rosaenz-Oroz, D.; Martinez-Ruiz, R.; Vaquero-Fernandez, L. Chemical and sensory characterisation of sweet wines obtained by different techniques. Cienc. e Tec. Vitivinic. 2018, 3I3, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Arena, E.; Rizzo, V.; Licciardello, F.; Fallico, B.; Muratore, G. Effects of light exposure, bottle colour and storage temperature on the quality of Malvasia delle Lipari sweet wine. Foods 2021, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Cetó, X.; Gutiérrez, J.M.; Gutiérrez, M.; Céspedes, F.; Capdevila, J.; Mínguez, S.; Jiménez-Jorquera, C.; del Vallea, M. Determination of total polyphenol index in wines employing a voltammetric electronic tongue. Anal. Chim. Acta 2012, 732, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcari, S.G.; Chaves, E.S.; Vanderlinde, R.; Rosier, J.P.; Bordignon-Luiz, M.T. Brazilian fortified wines: Chemical composition, chromatic properties and antioxidant activity. Food Res. Int. 2013, 53, 164–173. [Google Scholar] [CrossRef]
- Bekatorou, A. Alcohol: Properties and Determination. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 88–96. [Google Scholar]
- European Brewery Convention Analysis Committee. Analytica-EBC, 4th ed.; Brauerei und Getranke-Rundschau: Zurich, Switzerland, 1987. [Google Scholar]
- Murray, J.M.; Baxter, I.A. Sensory evaluation-Food acceptability and sensory evaluation. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 5130–5136. [Google Scholar]



| Parameter | SW-F | SW-S | SW-A |
|---|---|---|---|
| Total titratable acidity (g tartaric acid/L) | 7.39 ± 0.16 a | 7.35 ± 0.13 a | 6.38 ± 0.25 b |
| Volatile acidity (g acetic acid/L) | 0.32 ± 0.01 a | 0.27 ± 0.03 b | 0.67 ± 0.09 c |
| pH | 3.88 ± 0.02 a | 3.86 ± 0.02 a | 3.83 ± 0.02 b |
| Ethanol (% v/v) | 15.3 ± 0.1 a | 15.0 ± 0.5 a | 16.0 ± 0.4 b |
| Total sugars (g/L) | 121.7 ± 6.9 a | 138.6 ± 5.9 b | 110.7 ± 5.7 c |
| Glucose (g/L) | 59.2 ± 3.8 a | 67.4 ± 3.0 b | 44.2 ± 4.0 c |
| Fructose (g/L) | 62.5 ± 3.1 a | 71.2 ± 2.1 b | 66.5 ± 1.7 c |
| Saccharose (g/L) | nd | nd | nd |
| Free sulfite (mg/L) | 14.9 ± 3.2 a | 11.9 ± 0.8 a | 11.5 ± 1.0 a |
| Total sulfite (mg/L) | 24.3 ± 4.6 a | 29.4 ± 2.6 a | 44.8 ± 4.5 b |
| Citric acid (g/L) | 0.66 ± 0.01 a | 0.86 ± 0.15 b | 0.55 ± 0.09 c |
| Tartaric acid (g/L) | 2.19 ± 0.07 a | 2.58 ± 0.20 b | 1.99 ± 0.15 a |
| Malic acid (g/L) | 3.26 ± 0.05 a | 3.62 ± 0.04 b | 3.49 ± 0.07 c |
| Succinic acid (g/L) | 2.84 ± 0.03 a | 2.84 ± 0.09 a | 1.98 ± 0.12 b |
| Acetic acid (g/L) | 0.46 ± 0.31 a | 0.31 ± 0.44 | 0.48 ± 0.23 |
| Total phenolic content (mg GA/L) | 325 ± 5 a | 284 ± 4 b | 244 ± 5 c |
| Polyphenols (mg/L) | 576 ± 5 a | 555 ± 5 b | 459 ± 5 c |
| Antioxidant capacity (mg AA/L) | 35.0 ± 0.6 a | 24.0 ± 0.2 b | 18.0 ± 0.4 c |
| Compound | RID | RIref | RI | SW-F | SW-S | SW-A |
|---|---|---|---|---|---|---|
| Esters | ||||||
| Methyl acetate | A | 828 | 820.3 | 0.02 | <0.01 | <0.01 |
| Ethyl acetate | A | 888 | 882.3 | 10.48 | 8.73 | 5.03 |
| Ethyl propanoate | A | 953 | 949 | 0.08 | 0.05 | 0.04 |
| Ethyl 2-methylpropanoate (ethyl isobutyrate) | A | 961 | 958.2 | 0.08 | 0.06 | 0.04 |
| Propyl acetate | A | 973 | 967.8 | <0.01 | <0.01 | 0.01 |
| 2-Methylpropyl acetate (isobutyl acetate) | A | 1012 | 1012 | 0.13 | 0.08 | 0.13 |
| Ethyl butanoate (ethyl butyrate) | A | 1035 | 1034.7 | 0.51 | 0.24 | 0.43 |
| Ethyl 2-methylbutanoate (ethyl 2-methylbutyrate) | B | 1051 | 1050.5 | 0.06 | 0.06 | 0.01 |
| Ethyl 3-methylbutanoate (ethyl isovalerate) | B | 1068 | 1066.1 | 0.03 | 0.03 | <0.01 |
| Butyl acetate | A | 1074 | 1069.7 | <0.01 | <0.01 | <0.01 |
| 3-Methylbutyl acetate (isoamyl acetate) | A | 1122 | 1120 | 8.58 | 6.07 | 16.31 |
| Ethyl pentanoate (ethyl valerate) | A | 1134 | 1132.5 | 0.19 | 0.06 | 0.05 |
| 2-Methylpropyl butanoate (isobutyl butyrate) | B | 1158 | 1157.1 | <0.01 | <0.01 | <0.01 |
| Ethyl (E)-2-butenoate | B | 1160 | 1159.7 | 0.01 | 0.01 | 0.01 |
| Pentyl acetate (amyl acetate) | B | 1176 | 1171.2 | <0.01 | <0.01 | 0.01 |
| 3-Methylbutyl propanoate (isoamyl propanoate) | B | 1185 | 1187.2 | 0.02 | 0.02 | 0.01 |
| Butyl butanoate (butyl butyrate) | B | 1220 | 1216.4 | <0.01 | <0.01 | <0.01 |
| Ethyl hexanoate (ethyl caproate) | A | 1233 | 1231.5 | 10.07 | 7.35 | 15.51 |
| 3-Methylbutyl butanoate (isoamyl butyrate) | B | 1259 | 1264.9 | <0.01 | 0.01 | 0.02 |
| Hexyl acetate | A | 1272 | 1271.8 | <0.01 | <0.01 | 0.20 |
| Ethyl 5-hexenoate | C | 1271 | 1277.3 | <0.01 | <0.01 | <0.01 |
| Ethyl 3-hexenoate | C | 1290 | 1292.5 | 0.01 | 0.01 | <0.01 |
| Ethyl heptanoate (ethyl capronate) | B | 1331 | 1333.5 | 0.51 | 0.38 | 0.34 |
| Ethyl 2-hydroxypropanoate (ethyl lactate) | A | 1347 | 1343.7 | 0.03 | 0.03 | 0.01 |
| Heptyl acetate | C | 1377 | 1373.8 | <0.01 | <0.01 | <0.01 |
| Ethyl (E)-4-heptenoate | C | 1380 | 1374 | 0.01 | 0.03 | 0.05 |
| Ethyl octanoate (ethyl caprylate) | A | 1435 | 1434.7 | 10.66 | 14.84 | 23.56 |
| Ethyl 7-octenoate | C | 1478 | 1486.4 | 0.14 | 0.08 | 0.06 |
| Ethyl nonanoate (ethyl pelargonate) | A | 1531 | 1537.8 | 0.10 | 0.05 | 0.05 |
| Ethyl 2-hydroxy-4-methylpentanoate | C | 1547 | 1545.7 | 0.03 | 0.02 | 0.01 |
| Ethyl decanoate (ethyl caprate) | A | 1638 | 1639.9 | 0.67 | 3.49 | 6.04 |
| 3-Methylbutyl octanoate (isoamyl octanoate) | B | 1658 | 1661.7 | 0.02 | 0.04 | 0.04 |
| Diethyl butanedioate (Diethyl succinate) | A | 1680 | 1678 | 0.33 | 0.30 | 0.13 |
| Myrtenyl acetate (2-pinen-10-yl acetate) | C | 1698 | 1688.1 | <0.01 | <0.01 | <0.01 |
| Ethyl 9-decenoate | B | 1694 | 1692.5 | 0.26 | 0.49 | 1.19 |
| Ethyl 2-phenylacetate (ethyl benzeneacetate) | C | 1783 | 1781.9 | 0.04 | 0.05 | 0.01 |
| 2-Phenylethyl acetate | A | 1813 | 1811.2 | 0.27 | 0.43 | 1.28 |
| Ethyl dodecanoate (ethyl laurate) | A | 1841 | 1844.5 | 0.06 | 0.40 | 1.25 |
| 2-Phenylethyl propanoate | C | - | 1880.4 | <0.01 | <0.01 | <0.01 |
| Ethyl 3-phenylpropanoate (ethyl dihydrocinnamate) | C | 1893 | 1884 | 0.03 | 0.04 | 0.04 |
| 2-Phenylethyl butanoate (phenethyl butyrate) | B | 1958 | 1964.6 | <0.01 | <0.01 | 0.01 |
| Ethyl 3-methylbutyl butanedioate (Ethyl isopentyl succinate) | B | 1901 | 1904.7 | 0.03 | 0.04 | 0.01 |
| Octyl octanoate | B | 2009 | 2014.5 | 0.21 | 0.13 | 0.15 |
| Total | <43.67 | <43.61 | <72.06 | |||
| Alcohols | ||||||
| 1-Propanol | A | 1036 | 1042.6 | 0.06 | 0.05 | 0.03 |
| 2-Methyl-1-propanol (isobutanol) | A | 1092 | 1097.6 | 1.90 | 1.77 | 0.65 |
| 1-Butanol | A | 1142 | 1149.5 | 0.06 | 0.08 | <0.01 |
| 1-Penten-3-ol (ethyl vinyl carbinol) | B | 1159 | 1165.9 | <0.01 | <0.01 | <0.01 |
| 3-Methyl-1-butanol (isoamyl alcohol) | A | 1209 | 1212.8 | 37.23 | 36.77 | 16.88 |
| 1-Pentanol | A | 1250 | 1255 | 0.01 | 0.01 | 0.01 |
| 4-Methyl-1-pentanol (isohexyl alcohol) | A | 1314 | 1318.7 | 0.04 | 0.04 | 0.02 |
| (Z)-2-Penten-1-ol | B | 1318 | 1323.7 | <0.01 | <0.01 | <0.01 |
| 3-Methyl-1-pentanol | B | 1325 | 1331.4 | 0.05 | 0.05 | 0.03 |
| 1-Hexanol | A | 1355 | 1357.7 | 0.27 | 0.24 | 0.10 |
| (Z)-3-Hexen-1-ol | B | 1382 | 1386.8 | <0.01 | <0.01 | <0.01 |
| 3-Octanol | B | 1393 | 1398.8 | <0.01 | <0.01 | <0.01 |
| (E)-2-Hexen-1-ol | B | 1405 | 1409.2 | <0.01 | <0.01 | <0.01 |
| 2-Octanol | A | 1412 | 1425.5 | 0.03 | 0.03 | 0.02 |
| 1-Octen-3-ol | A | 1450 | 1454.2 | 0.47 | 0.23 | 0.16 |
| 1-Heptanol | B | 1453 | 1460 | 0.41 | 0.35 | 0.38 |
| 2-Ethyl-1-hexanol | A | 1491 | 1493.5 | 0.06 | 0.08 | 0.02 |
| (E)-2-Hepten-1-ol | C | 1517 | 1514.2 | <0.01 | <0.01 | <0.01 |
| 2-Nonanol | C | 1521 | 1525.1 | 0.03 | 0.04 | 0.01 |
| 2,3-Butanediol isomer 1 | C | 1543 | 1544.3 | 0.06 | 0.09 | 0.03 |
| 1-Octanol | A | 1557 | 1562.5 | 0.48 | 0.43 | 0.18 |
| 2,3-Butanediol isomer 2 | C | 1556 | 1581.4 | 0.02 | 0.04 | 0.01 |
| (E)-2-Octen-1-ol | C | 1614 | 1616.6 | 0.07 | 0.02 | 0.02 |
| 2-Furanmethanol (Furfuryl alcohol) | B | 1660 | 1661.3 | <0.01 | <0.01 | <0.01 |
| 1-Nonanol | B | 1660 | 1664.9 | 0.22 | 0.27 | 0.09 |
| 3-(Methylthio)-1-propanol (methionol) | B | 1719 | 1718.3 | <0.01 | <0.01 | <0.01 |
| 2-Dodecanol | C | 1813 | 1821.8 | 0.01 | 0.01 | <0.01 |
| Phenylmethanol (benzyl alcohol) | B | 1870 | 1875.4 | <0.01 | 0.01 | <0.01 |
| 2-Phenylethanol (phenylethyl alcohol) | A | 1906 | 1912.2 | 8.41 | 9.92 | 5.45 |
| 1-Dodecanol (lauryl alcohol) | B | 1966 | 1972.4 | 0.46 | 0.24 | 0.13 |
| 1-Tetradecanol (myristyl alcohol) | C | 2165 | 2181.5 | 0.33 | 0.05 | 0.04 |
| Total | <50.70 | <50.83 | <24.25 | |||
| Organic acids | ||||||
| Acetic acid | A | 1449 | 1448.6 | 0.51 | 0.84 | 0.31 |
| Propanoic acid | B | 1535 | 1538.2 | <0.01 | <0.01 | <0.01 |
| 2-Methylpropanoic acid (isobutyric acid) | C | 1570 | 1569.2 | 0.02 | 0.02 | <0.01 |
| Butanoic acid | B | 1625 | 1628.5 | <0.01 | <0.01 | <0.01 |
| 3-Methylbutanoic acid (isovaleric acid) | B | 1666 | 1671 | 0.03 | 0.03 | 0.01 |
| 2-Methylbutanoic acid | C | 1662 | 1672.2 | 0.01 | 0.02 | 0.01 |
| Pentanoic acid (valeric acid) | B | 1733 | 1737.4 | <0.01 | <0.01 | <0.01 |
| Hexanoic acid (caproic acid) | A | 1846 | 1844.6 | 0.16 | 0.16 | 0.19 |
| 3-Methylhexanoic acid | C | - | 1955 | <0.01 | <0.01 | <0.01 |
| Octanoic acid (caprylic acid) | A | 2060 | 2062.5 | 0.45 | 0.69 | 1.16 |
| Nonanoic acid | C | 2171 | 2174.3 | 0.01 | <0.01 | 0.01 |
| n-Decanoic acid (capric acid) | Β | 2276 | 2250.8 | 0.05 | 0.31 | 0.59 |
| Total | <1.25 | <2.07 | <2.28 | |||
| Carbonyl compounds | ||||||
| Acetaldehyde | A | 702 | 698.3 | 0.27 | 0.26 | 0.22 |
| 2-Methylpropanal (isobutyraldehyde) | B | 819 | 807.9 | <0.01 | <0.01 | <0.01 |
| Butanal (butyraldehyde) | B | 877 | 867.3 | <0.01 | <0.01 | <0.01 |
| 2-Butanone (methyl ethyl ketone) | B | 907 | 899.3 | <0.01 | <0.01 | <0.01 |
| 2-Methylbutanal | B | 914 | 908.1 | 0.02 | 0.01 | <0.01 |
| 3-Methylbutanal (isovaleraldehyde) | B | 918 | 911.4 | 0.13 | 0.07 | 0.02 |
| 2,3-Butanedione (Diacetyl) | A | 979 | 968.3 | 0.03 | 0.02 | <0.01 |
| Hexanal | A | 1083 | 1076.1 | 0.69 | 0.31 | 0.09 |
| 2-Heptanone | B | 1182 | 1177.7 | 0.01 | <0.01 | <0.01 |
| Heptanal (oenanthic aldehyde) | B | 1184 | 1179 | 0.06 | 0.04 | 0.01 |
| 4-Methyl-2-heptanone | B | 1206 | 1203.4 | <0.01 | <0.01 | <0.01 |
| 3-Octanone (ethyl amyl ketone) | B | 1253 | 1251.7 | 0.03 | 0.0 | 0.01 |
| 3-Hydroxy-2-butanone (acetoin) | A | 1284 | 1280.6 | 0.03 | 0.01 | <0.01 |
| Octanal | B | 1289 | 1284.2 | 0.49 | 0.51 | 0.08 |
| 2-Heptenal | B | 1323 | 1318.6 | 0.04 | 0.04 | 0.01 |
| 6-Methyl-5-hepten-2-one | C | 1338 | 1334.8 | 0.11 | 0.08 | 0.06 |
| 2-Nonanone | C | 1390 | 1387.7 | 0.09 | 0.06 | 0.01 |
| Nonanal | B | 1391 | 1390.5 | 0.20 | 0.09 | 0.05 |
| 3-Octen-2-one | C | 1411 | 1404.5 | 0.04 | <0.01 | <0.01 |
| (E)-2-Octenal | C | 1429 | 1425.8 | 0.08 | 0.12 | 0.07 |
| 2-Furfuraldehyde (furfural) | A | 1461 | 1459.2 | 0.51 | 0.51 | 0.14 |
| Decanal | B | 1498 | 1498.2 | 0.01 | 0.01 | <0.01 |
| Phenylmethanal (benzaldehyde) | A | 1520 | 1516.8 | 0.70 | 0.50 | 0.04 |
| (E)-2-Nonenal | C | 1534 | 1533.4 | <0.01 | <0.01 | <0.01 |
| (3E,5E)-3,5-Octadien-2-one | C | 1570 | 1568.8 | <0.01 | <0.01 | <0.01 |
| 5-Methyl-2-furfural | B | 1570 | 1569.5 | <0.01 | <0.01 | <0.01 |
| 6-Methyl-3,5-heptadiene-2-one | B | 1602 | 1591.1 | <0.01 | <0.01 | <0.01 |
| Ethyl-1H-pyrrole-2-carboxaldehyde | C | 1610 | 1605.3 | <0.01 | 0.01 | 0.01 |
| Phenylacetaldehyde | C | 1640 | 1636.2 | <0.01 | <0.01 | <0.01 |
| 2,4-Nonadienal | C | 1700 | 1699.7 | <0.01 | <0.01 | <0.01 |
| 2,4-Decadienal | B | 1797 | 1805.2 | 0.01 | 0.01 | <0.01 |
| 1H-Pyrrole-2-carboxaldehyde (pyrrole aldehyde) | B | 2030 | 2022.9 | <0.01 | <0.01 | <0.01 |
| Total | <3.55 | <2.71 | <0.82 | |||
| Terpenes | ||||||
| d-Limonene (1-methyl-4-prop-1-en-2-ylcyclohexene) | A | 1200 | 1185.4 | 0.08 | 0.04 | 0.10 |
| trans-Rose oxide [tetrahydro-4-methyl-2-(2-methylpropenyl)-2H-pyran] | C | 1365 | 1351.4 | <0.01 | 0.01 | <0.01 |
| Verbenyl ethyl ether (4-ethoxy-2,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene) | C | 1377 | 1372.5 | <0.01 | <0.01 | <0.01 |
| Thujone [(1S,4S,5R)-4-methyl-1-propan-2-ylbicyclo[3.1.0]hexan-3-one] | B | 1430 | 1416.6 | 0.04 | 0.02 | 0.02 |
| Linalool (3,7-dimethyl-1,6-octadien-3-ol) | A | 1547 | 1550.9 | 0.03 | 0.03 | 0.01 |
| Fenchol (1,3,3-trimethylbicyclo[2.2.1]heptan-2-ol) | B | 1582 | 1585.6 | <0.01 | <0.01 | <0.01 |
| L-4-Terpineol (4-methyl-1-propan-2-ylcyclohex-3-en-1-ol) | B | 1593 | 1604.1 | <0.01 | <0.01 | <0.01 |
| β-Cyclocitral (2,6,6-trimethylcyclohexene-1-carbaldehyde) | C | 1611 | 1618.2 | <0.01 | <0.01 | <0.01 |
| α-Terpineol [2-(4-methyl-3-cyclohexen-1-yl)-2-propanol] | A | 1697 | 1700.5 | 0.02 | 0.01 | <0.01 |
| l-Borneol (1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-ol) | B | 1702 | 1704.1 | <0.01 | <0.01 | <0.01 |
| β-Citronellol (3,7-dimethyl-6-octenol) | A | 1765 | 1767.7 | 0.06 | 0.12 | 0.03 |
| Nerol [(Z)-3,7-dimethyl-2,6-octadien-1-ol] | B | 1797 | 1799.8 | <0.01 | <0.01 | <0.01 |
| β-Damascenone | C | 1823 | 1817.9 | 0.02 | 0.03 | 0.02 |
| Geraniol (trans-3,7-dimethyl-2,7-octadien-1-ol) | A | 1847 | 1849.6 | <0.01 | <0.01 | 0.01 |
| trans-Geranylacetone [(E)-6,10-dimethylundeca-5,9-dien-2-one] | C | 1859 | 1853.9 | 0.02 | 0.02 | 0.01 |
| trans-β-Ionone [(E)-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one] | C | 1940 | 1940.7 | <0.01 | <0.01 | <0.01 |
| Epicubenol [1S,4R,4aS,8aR)-4,7-dimethyl-1-propan-2-yl-2,3,4,5,6,8a-hexahydro-1H-naphthalen-4a-ol] | C | 2067 | 2070 | <0.01 | <0.01 | <0.01 |
| T-Muurolol [(1S,4S,4aR,8aS)-1,6-dimethyl-4-propan-2-yl-3,4,4a,7,8,8a-hexahydro-2H-naphthalen-1-ol] | C | 2186 | 2194.1 | <0.01 | <0.01 | <0.01 |
| α-Cadinol [(1R,4S,4aR)-1,6-dimethyl-4-propan-2-yl-3,4,4a,7,8,8a-hexahydro-2H-naphthalen-1-ol] | C | 2226 | 2228.4 | <0.01 | <0.01 | <0.01 |
| Total | <0.29 | <0.28 | <0.21 | |||
| Lactones | ||||||
| Dihydrofuran-2(3H)-one (γ-Butyrolactone) | B | 1632 | 1622.2 | 0.04 | 0.03 | 0.01 |
| 5-Methyl-2(5H)-furanone (β-Angelica lactone) | C | 1669 | 1673.6 | <0.01 | <0.01 | <0.01 |
| Dihydro-5-ethyl-2(3H)-furanone (γ-hexalactone) | C | 1694 | 1698.2 | <0.01 | <0.01 | <0.01 |
| 5-Ethyl-2(5H)-furanone (2-hexen-1,4-lactone) | C | 1745 | 1753.1 | <0.01 | <0.01 | <0.01 |
| 6-Propyl tetrahydro-2H-pyran-2-one (δ-Octalactone) | C | 1976 | 1978.4 | 0.05 | 0.08 | 0.03 |
| Dihydro-5-pentyl-2(3H)-furanone (γ-Nonalactone) | C | 2024 | 2028.8 | 0.02 | 0.05 | 0.03 |
| Total | <0.12 | <0.16 | <0.07 | |||
| Other compounds | ||||||
| Dimethyl sulfide | B | 754 | 738.9 | <0.01 | <0.01 | <0.01 |
| 1,1-Diethoxy ethane (Acetal) | B | 892 | 890.8 | 0.25 | 0.24 | 0.26 |
| 2-Ethylfuran | B | 950 | 942.6 | <0.01 | <0.01 | <0.01 |
| 1,4-Dioxane (IS) | 1055.5 | |||||
| 2-Pentylfuran | B | 1231 | 1225.6 | 0.02 | 0.02 | 0.01 |
| 2-Acetylfuran | B | 1499 | 1500.4 | 0.02 | 0.02 | <0.01 |
| Methyl eugenol (1,2-dimethoxy-4-prop-2-enylbenzene) | C | 2013 | 2013.2 | <0.01 | <0.01 | <0.01 |
| Total | <0.29 | <0.29 | <0.27 | |||
| Hydrocarbons (alkanes/alkenes) | ||||||
| Hexane | A | 600 | 600 | <0.01 | 0.01 | <0.01 |
| Heptane | A | 700 | 700 | <0.01 | <0.01 | <0.01 |
| Octane | A | 800 | 800 | <0.01 | <0.01 | <0.01 |
| 1-Octene | C | 847 | 831.8 | <0.01 | <0.01 | <0.01 |
| 2-Octene | C | 864 | 848.4 | <0.01 | <0.01 | <0.01 |
| Nonane | A | 900 | 900 | <0.01 | <0.01 | <0.01 |
| Decane | A | 1000 | 1000 | <0.01 | <0.01 | <0.01 |
| Dodecane | A | 1200 | 1200 | <0.01 | <0.01 | <0.01 |
| Tetradecane | A | 1400 | 1400 | <0.01 | <0.01 | <0.01 |
| Hexadecane | A | 1600 | 1600 | <0.01 | <0.01 | <0.01 |
| Naphthalene | C | 1746 | 1734 | 0.01 | <0.01 | 0.03 |
| Total | <0.03 | <0.01 | <0.04 |
| Descriptor | Description/Average Score | |||||
|---|---|---|---|---|---|---|
| SW-F | SW-S | SW-A | ||||
| Appearance | ||||||
| Clarity | Clear | 9 | Clear | 9 | Clear | 9 |
| Color intensity | Medium | 5 | Deep | 9 | Medium | 5 |
| Color description | Brown amber | Brown amber | Brown amber | |||
| Sediment | no | 0 | no | 0 | no | 0 |
| Nose | ||||||
| Aroma intensity | Medium | 6 | Medium | 5 | Medium | 6 |
| Aroma description | Fruity, raisin, grape | Fruity, raisin, grape | Fruity, raisin, grape | |||
| Palate | ||||||
| Sweetness | Sweet | 9 | Sweet | 9 | Sweet | 6 |
| Acidity | Medium | 6 | Medium | 5 | Medium | 5 |
| Tannin | Medium | 4 | Medium | 4 | Low | 2 |
| Alcohol | Strong | 6 | Strong | 6 | Strong | 6 |
| Body | Medium | 5 | Medium | 6 | Medium | 4 |
| Taste intensity | High | 8 | High | 8 | Medium | 5 |
| Taste description | Sweet, raisin | Sweet, raisin | Sweet, light fruity | |||
| Aftertaste | Medium | 6 | Medium | 6 | Medium | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plioni, I.; Michalopoulou, E.; Mallouchos, A.; Plessas, S.; Gotis, G.; Bekatorou, A. Sweet Wine Production from the Side-Stream of Industrial Corinthian Currant Processing: Product Quality, Antioxidant Capacity, and Volatilome. Molecules 2023, 28, 5458. https://doi.org/10.3390/molecules28145458
Plioni I, Michalopoulou E, Mallouchos A, Plessas S, Gotis G, Bekatorou A. Sweet Wine Production from the Side-Stream of Industrial Corinthian Currant Processing: Product Quality, Antioxidant Capacity, and Volatilome. Molecules. 2023; 28(14):5458. https://doi.org/10.3390/molecules28145458
Chicago/Turabian StylePlioni, Iris, Eleni Michalopoulou, Athanasios Mallouchos, Stavros Plessas, Gerasimos Gotis, and Argyro Bekatorou. 2023. "Sweet Wine Production from the Side-Stream of Industrial Corinthian Currant Processing: Product Quality, Antioxidant Capacity, and Volatilome" Molecules 28, no. 14: 5458. https://doi.org/10.3390/molecules28145458