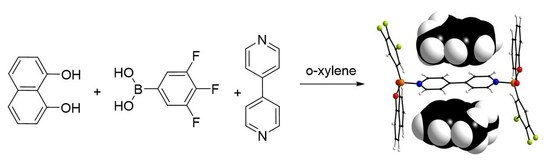
1,8-Dihydroxy Naphthalene—A New Building Block for the Self-Assembly with Boronic Acids and 4,4′-Bipyridine to Stable Host–Guest Complexes with Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Brunet, P.; Simard, M.; Wuest, J.D. Molecular tectonics. Porous hydrogen-bonded networks with unprecedented structural integrity. J. Am. Chem. Soc. 1997, 119, 2737–2738. [Google Scholar] [CrossRef]
- Hosseini, M.W. Molecular tectonics: From simple tectons to complex molecular networks. Acc. Chem. Res. 2005, 38, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, M. Shape-Persistent Organic Cage Compounds by Dynamic Covalent Bond Formation. Angew. Chem. Int. Ed. 2010, 49, 5042–5053. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar]
- Smulders, M.M.J.; Riddell, I.A.; Browne, C.; Nitschke, J.R. Building on architectural principles for three-dimensional metallosupramolecular construction. Chem. Soc. Rev. 2013, 42, 1728–1754. [Google Scholar] [CrossRef]
- Goesten, M.G.; Kapteijn, F.; Gascon, J. Fascinating chemistry or frustrating unpredictability: Observations in crystal engineering of metal−organic frameworks. CrystEngComm 2013, 15, 9249–9257. [Google Scholar] [CrossRef] [Green Version]
- Harris, K.; Fujita, D.; Fujita, M. Giant hollow MnL2n spherical complexes: Structure, functionalisation and applications. Chem. Commun. 2013, 49, 6703–6712. [Google Scholar] [CrossRef]
- Song, M.; Sun, Z.; Han, C.; Tian, D.; Li, H.; Kim, J.S. Calixarene based chemosensors by means of click chemistry. Chem. Asian J. 2014, 9, 2344–2357. [Google Scholar] [CrossRef]
- Durola, F.; Heitz, V.; Reviriego, F.; Roche, C.; Sauvage, J.-P.; Sour, A.; Trolez, Y. Cyclic [4]rotaxanes containing two parallel porphyrinic plates: Toward switchable molecular receptors and Compressors. Acc. Chem. Res. 2014, 47, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Mastalerz, M. Organic cage compounds—From shape-persistency to function. Chem. Soc. Rev. 2014, 43, 1934–1947. [Google Scholar] [CrossRef]
- Li, H.; Yao, Z.-J.; Liu, D.; Jin, G.-X. Multi-component coordination-driven self-assembly toward heterometallic macrocycles and cages. Coord. Chem. Rev. 2015, 293–294, 139–157. [Google Scholar]
- Torres-Huerta, A.; Velásquez-Hernández, M.; Martínez-Otero, D.; Höpfl, H.; Jancik, V. Structural induction via solvent variation in assemblies of triphenylboroxine and piperazine—Potential application as self-assembly molecular sponge. Cryst. Growth Des. 2017, 17, 2438–2452. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Du, F.; Wang, G.-D.; Hou, L.; Zhu, Z.; Liu, B.; Wang, Y.-Y. Dative B←N bonds based crystalline organic framework with permanent porosity for acetylene storage and separation. Chem. Sci. 2023, 14, 533–539. [Google Scholar]
- Stephens, A.J.; Scopelliti, R.; Tirani, F.F.; Solari, E.; Severin, K. Crystalline Polymers Based on Dative Boron-Nitrogen Bonds and the Quest for Porosity. ACS Mater. Lett. 2019, 1, 3–7. [Google Scholar] [CrossRef]
- Bhandary, S.; Shukla, R.; Van Hecke, K. Effect of chemical substitution on the construction of boroxine-based supramolecular crystalline polymers featuring B←N dative bonds. CrystEngComm 2022, 24, 1695–1699. [Google Scholar] [CrossRef]
- Madura, I.D.; Czerwinska, K.; Jakubczyk, M.; Pawelko, A.; Adamczyk-Wozniak, A.; Sporzynski, A. Weak C–H···O and Dipole–Dipole Interactions as Driving Forces in Crystals of Fluorosubstituted Phenylboronic Catechol Esters. Cryst. Growth Des. 2013, 13, 5344–5352. [Google Scholar] [CrossRef]
- Luisier, N.; Scopelliti, R.; Severin, K. Supramolecular gels based on boronate esters and imidazolyl donors. Soft Matter 2016, 12, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Mendoza, D.; Cruz-Huerta, J.; Höpfl, H.; Hernández-Ahuactzi, I.F.; Sanchez, M. Macrocycles and coordination polymers derived from self-complementary tectons based on N-containing boronic acids. Cryst. Growth Des. 2013, 13, 2441–2454. [Google Scholar] [CrossRef]
- Ray, K.K.; Campillo-Alvarado, G.; Morales-Rojas, H.; Hopfl, H.; MacGillivray, L.R.; Tivanski, A.V. Semiconductor Cocrystals Based on Boron: Generated Electrical Response with π-Rich Aromatic Molecules. Cryst. Growth Des. 2020, 20, 3–8. [Google Scholar] [CrossRef]
- Icli, B.; Solari, E.; Kilbas, B.; Scopelliti, R.; Severin, K. Multicomponent Assembly of Macrocycles and Polymers by Coordination of Pyridyl Ligands to 1,4-Bis(benzodioxaborole)benzene. Chem. Eur. J. 2012, 18, 14867–14874. [Google Scholar] [CrossRef]
- Christinat, N.; Scopelliti, R.; Severin, K. Boron-based rotaxanes by multicomponent self-assembly. Chem. Commun. 2008, 3660–3662. [Google Scholar] [CrossRef] [PubMed]
- Hartwick, C.J.; Yelgaonkar, S.P.; Reinheimer, E.W.; Campillo-Alvarado, G.; MacGillivray, L.R. Self-Assembly of Diboronic Esters with U-Shaped Bipyridines: “Plugin-Socket” Assemblies. Cryst. Growth Des. 2021, 21, 4482–4487. [Google Scholar] [CrossRef] [PubMed]
- Christinat, N.; Croisier, E.; Scopelliti, R.; Cascella, M.; Rothlisberger, U.; Severin, K. Formation of boronate ester polymers with efficient intrastrand charge-transfer transitions by three-component reactions. Eur. J. Inorg. Chem. 2007, 2007, 5177–5181. [Google Scholar] [CrossRef]
- Herrera-España, A.D.; Campillo-Alvarado, G.; Román-Bravo, P.; Herrera-Ruiz, D.; Höpfl, H.; Morales-Rojas, H. Selective Isolation of Polycyclic Aromatic Hydrocarbons by Self-Assembly of a Tunable N→B Clathrate. Cryst. Growth Des. 2015, 15, 1572–1576. [Google Scholar] [CrossRef]
- Dhara, A.; Beuerle, F. Reversible Assembly of a Supramolecular Cage Linked by Boron–Nitrogen Dative Bonds. Chem. Eur. J. 2015, 21, 17391–17396. [Google Scholar] [CrossRef]
- Icli, B.; Sheepwash, E.; Riis-Johannessen, T.; Schenk, K.; Filinchuk, Y.; Scopelliti, R.; Severin, K. Dative boron–nitrogen bonds in structural supramolecular chemistry: Multicomponent assembly of prismatic organic cages. Chem. Sci. 2011, 2, 1719–1721. [Google Scholar]
- Campillo-Alvarado, G.; Vargas-Olvera, E.C.; Hopfl, H.; Herrera-Espana, A.D.; Sanchez-Guadarrama, O.; Morales-Rojas, H.; MacGillivray, L.R.; Rodriguez-Molina, B.; Farfan, N. Self-Assembly of Fluorinated Boronic Esters and 4,4′-Bipyridine into 2:1 N→B Adducts and Inclusion of Aromatic Guest Molecules in the Solid State: Application for the Separation of o,m,p-Xylene. Cryst. Growth Des. 2018, 18, 2726–2743. [Google Scholar] [CrossRef]
- Herrera-Espana, A.D.; Hoepfl, H.; Morales-Rojas, H. Boron-Nitrogen Double Tweezers Comprising Arylboronic Esters and Diamines: Self-Assembly in Solution and Adaptability as Hosts for Aromatic Guests in the Solid State. ChemPlusChem 2020, 85, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Manankandayalage, C.P.; Unruh, D.K.; Krempner, C. Boronic, diboronic and boric acid esters of 1,8-naphthalenediol–synthesis, structure and formation of boronium salts. Dalton Trans. 2020, 49, 4834–4842. [Google Scholar] [CrossRef]
- Bruker (2021) SADABS v2016/2, Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 9–18. [Google Scholar] [CrossRef] [Green Version]
- CrysAlisPRO. Oxford Diffraction; Agilent Technologies UK Ltd.: Yarnton, UK, 2018. [Google Scholar]
- SCALE3 ABSPACK, Oxford Diffraction Ltd.: Abingdon, UK, 2005.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]










| 2d × Solvent | 2d/Solvent Ratio (from X-ray Data) | 2d/Solvent Ratio (from 1H NMR Data) 1 |
|---|---|---|
| 2d × benzene | 2:3 | 1:1.5 |
| 2d × toluene | 1:1 | 1:1 |
| 2d × o-xylene | 1:2 | 1:1 |
| 2d × m-xylene 2 | 3:6 3 and 1:1 | 1:1 |
| 2d × p-xylene | 1:1 | 1:1 |
| 2d × mesitylene | 1:2 4 | 1:2.66 |
| 2d × aniline | 2:3 | 1:1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manankandayalage, C.P.; Unruh, D.K.; Perry, R.; Krempner, C. 1,8-Dihydroxy Naphthalene—A New Building Block for the Self-Assembly with Boronic Acids and 4,4′-Bipyridine to Stable Host–Guest Complexes with Aromatic Hydrocarbons. Molecules 2023, 28, 5394. https://doi.org/10.3390/molecules28145394
Manankandayalage CP, Unruh DK, Perry R, Krempner C. 1,8-Dihydroxy Naphthalene—A New Building Block for the Self-Assembly with Boronic Acids and 4,4′-Bipyridine to Stable Host–Guest Complexes with Aromatic Hydrocarbons. Molecules. 2023; 28(14):5394. https://doi.org/10.3390/molecules28145394
Chicago/Turabian StyleManankandayalage, Chamila P., Daniel K. Unruh, Ryan Perry, and Clemens Krempner. 2023. "1,8-Dihydroxy Naphthalene—A New Building Block for the Self-Assembly with Boronic Acids and 4,4′-Bipyridine to Stable Host–Guest Complexes with Aromatic Hydrocarbons" Molecules 28, no. 14: 5394. https://doi.org/10.3390/molecules28145394