Bifunctional Hot Water Vapor Template-Mediated Synthesis of Nanostructured Polymeric Carbon Nitride for Efficient Hydrogen Evolution
Abstract
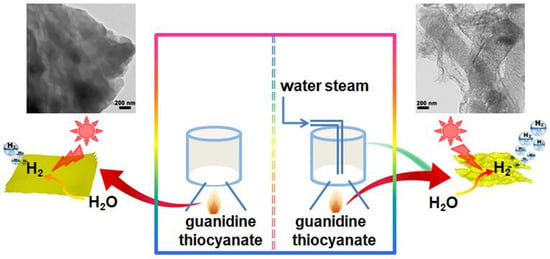
:1. Introduction
2. Results and Discussion
2.1. Morphology and Texture
2.2. XRD and FTIR Analysis
2.3. XPS and ESR Analysis
2.4. UV-Visible and PL Analysis
2.5. Time-Resolved PL and Photoelectrochemical Analysis
2.6. Photocatalytic Activities
2.7. Photocatalytic Activities of the Other Prepared Nanostructured PCN
3. Materials and Methods
3.1. Materials
3.2. Preparation
3.3. Characterization
3.4. Photocatalytic H2 Evolution Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef]
- Meng, S.; Chen, C.; Gu, X.; Wu, H.; Meng, Q.; Zhang, J.; Chen, S.; Fu, X.; Liu, D.; Lei, W. Efficient photocatalytic H2 evolution, CO2 reduction and N2 fixation coupled with organic synthesis by cocatalyst and vacancies engineering. Appl. Catal. B 2021, 285, 119789. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Liang, X.; Xue, S.; Yang, C.; Ye, X.; Wang, Y.; Chen, Q.; Lin, W.; Hou, Y.; Zhang, G.; Shalom, M.; et al. The Directional Crystallization Process of Poly (triazine imide) Single Crystals in Molten Salts. Angew. Chem. Int. Ed. 2023, 62, e202216434. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Mullins, C.B. Understanding Charge Transport in Carbon Nitride for Enhanced Photocatalytic Solar Fuel Production. Acc. Chem. Res. 2019, 52, 248–257. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lin, Z.; Zhang, J.; Cai, X.; Lin, W.; Yu, Z.; Wang, X. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 2020, 3, 649–655. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Huang, F.; Song, S.; Ai, G.; Xin, X.; Zhao, B.; Zheng, Y.; Zhang, Z. Recent Advances in g-C3N4-Based Materials and Their Application in Energy and Environmental Sustainability. Molecules 2023, 28, 432. [Google Scholar] [CrossRef]
- Aljuaid, A.; Almehmadi, M.; Alsaiari, A.A.; Allahyani, M.; Abdulaziz, O.; Alsharif, A.; Alsaiari, J.A.; Saih, M.; Alotaibi, R.T.; Khan, I. g-C3N4 Based Photocatalyst for the Efficient Photodegradation of Toxic Methyl Orange Dye: Recent Modifications and Future Perspectives. Molecules 2023, 28, 3199. [Google Scholar] [CrossRef]
- Cao, D.; Wang, X.; Zhang, H.; Yang, D.; Yin, Z.; Liu, Z.; Lu, C.; Guo, F. Rational Design of Monolithic g-C3N4 with Floating Network Porous-like Sponge Monolithic Structure for Boosting Photocatalytic Degradation of Tetracycline under Simulated and Natural Sunlight Illumination. Molecules 2023, 28, 3989. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Kessler, F.K.; Zheng, Y.; Schwarz, D.; Merschjann, C.; Schnick, W.; Wang, X.; Bojdys, M.J. Functional carbon nitride materials- design strategies for electrochemical devices. Nat. Rev. Mater. 2017, 2, 17030. [Google Scholar] [CrossRef]
- Xiao, X.; Gao, Y.; Zhang, L.; Zhang, J.; Zhang, Q.; Li, Q.; Bao, H.; Zhou, J.; Miao, S.; Chen, N.; et al. A Promoted Charge Separation/Transfer System from Cu Single Atoms and C3N4 Layers for Efficient Photocatalysis. Adv. Mater. 2020, 32, 2003082. [Google Scholar] [CrossRef]
- Lin, J.; Tian, W.; Guan, Z.; Zhang, H.; Duan, X.; Wang, H.; Sun, H.; Fang, Y.; Huang, Y.; Wang, S. Functional Carbon Nitride Materials in Photo-Fenton-Like Catalysis for Environmental Remediation. Adv. Funct. Mater. 2022, 32, 2201743. [Google Scholar] [CrossRef]
- Yang, P.; Ou, H.; Fang, Y.; Wang, X. A Facile Steam Reforming Strategy to Delaminate Layered Carbon Nitride Semiconductors for Photoredox Catalysis. Angew. Chem. Int. Ed. 2017, 56, 3992–3996. [Google Scholar] [CrossRef]
- Yang, P.; Zhuzhang, H.; Wang, R.; Lin, W.; Wang, X. Carbon Vacancies in a Melon Polymeric Matrix Promote Photocatalytic Carbon Dioxide Conversion. Angew. Chem. Int. Ed. 2019, 58, 1134–1137. [Google Scholar] [CrossRef]
- Niu, P.; Yin, L.-C.; Yang, Y.-Q.; Liu, G.; Cheng, H.-M. Increasing the Visible Light Absorption of Graphitic Carbon Nitride (Melon) Photocatalysts by Homogeneous Self-Modification with Nitrogen Vacancies. Adv. Mater. 2014, 26, 8046–8052. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Singh, G.; Kim, I.Y.; Albahily, K.; Al-Muhtaseb, A.a.H.; Karakoti, A.S.; Tavakkoli, E.; Vinu, A. Nanostructured Carbon Nitrides for CO2 Capture and Conversion. Adv. Mater. 2020, 32, 1904635. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; Du, X.; Dong, F.; Zhang, Y. Rational nanostructure design of graphitic carbon nitride for photocatalytic applications. J. Mater. Chem. A 2019, 7, 11584–11612. [Google Scholar] [CrossRef]
- Chen, L.; Song, J. Tailored Graphitic Carbon Nitride Nanostructures: Synthesis, Modification, and Sensing Applications. Adv. Funct. Mater. 2017, 27, 1702695. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Wang, X. Nanostructured Carbon Nitrides for Photocatalytic Water Splitting. In Nanocarbons for Advanced Energy Conversion, 1st ed.; Feng, X.L., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2015; pp. 281–300. [Google Scholar]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Kröger, J.; Jiménez-Solano, A.; Savasci, G.; Lau, V.W.H.; Duppel, V.; Moudrakovski, I.; Küster, K.; Scholz, T.; Gouder, A.; Schreiber, M.-L.; et al. Morphology Control in 2D Carbon Nitrides: Impact of Particle Size on Optoelectronic Properties and Photocatalysis. Adv. Funct. Mater. 2021, 31, 2102468. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K. State-of-the-art review of morphological advancements in graphitic carbon nitride (g-CN) for sustainable hydrogen production. Renew. Sustain. Energy Rev. 2021, 135, 110235. [Google Scholar] [CrossRef]
- Long, B.; Zheng, Y.; Lin, L.; Alamry, K.A.; Asiri, A.M.; Wang, X. Cubic mesoporous carbon nitride polymers with large cage-type pores for visible light photocatalysis. J. Mater. Chem. A 2017, 5, 16179–16188. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, X.; Zhang, C.; Lin, L.; Xing, W.; Yu, Z.; Zhang, G.; Wang, X. Improved Charge Separation in Poly(heptazine-triazine) Imides with Semi-coherent Interfaces for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202210849. [Google Scholar]
- Huang, D.; Yan, X.; Yan, M.; Zeng, G.; Zhou, C.; Wan, J.; Cheng, M.; Xue, W. Graphitic Carbon Nitride-Based Heterojunction Photoactive Nanocomposites: Applications and Mechanism Insight. ACS Appl. Mater. Interfaces 2018, 10, 21035–21055. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Sun, R.-Q.; Wang, X. A Facile Band Alignment of Polymeric Carbon Nitride Semiconductors to Construct Isotype Heterojunctions. Angew. Chem. Int. Ed. 2012, 51, 10145–10149. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A Hierarchical Z-Scheme α-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef]
- Chen, S.; Wei, J.; Ren, X.; Song, K.; Sun, J.; Bai, F.; Tian, S. Recent Progress in Porphyrin/g-C3N4 Composite Photocatalysts for Solar Energy Utilization and Conversion. Molecules 2023, 28, 4283. [Google Scholar] [CrossRef]
- Iqbal, W.; Yang, B.; Zhao, X.; Rauf, M.; Waqas, M.; Gong, Y.; Zhang, J.; Mao, Y. Controllable synthesis of graphitic carbon nitride nanomaterials for solar energy conversion and environmental remediation: The road travelled and the way forward. Catal. Sci. Technol. 2018, 8, 4576–4599. [Google Scholar] [CrossRef]
- Martin, D.J.; Qiu, K.; Shevlin, S.A.; Handoko, A.D.; Chen, X.; Guo, Z.; Tang, J. Highly Efficient Photocatalytic H2 Evolution from Water using Visible Light and Structure-Controlled Graphitic Carbon Nitride. Angew. Chem. Int. Ed. 2014, 53, 9240–9245. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, J.; Maeda, K.; Domen, K.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Sulfur-mediated synthesis of carbon nitride: Band-gap engineering and improved functions for photocatalysis. Energy Environ. Sci. 2011, 4, 675–678. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Long, B.; Lin, J.; Wang, X. Thermally-induced desulfurization and conversion of guanidine thiocyanate into graphitic carbon nitride catalysts for hydrogen photosynthesis. J. Mater. Chem. A 2014, 2, 2942–2951. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Wang, H.; Cao, F.; Chen, F. Polycondensation of ammonium thiocyanate into novel porous g-C3N4 nanosheets as photocatalysts for enhanced hydrogen evolution under visible light irradiation. Chin. J. Catal. 2016, 37, 1899–1906. [Google Scholar] [CrossRef]
- Madhurima, V.P.; Kumari, K.; Jain, P.K. A facile single-step approach to achieve in situ expanded g-C3N4 for improved photodegradation performance. Polym. Adv. Technol. 2023, 34, 578–586. [Google Scholar] [CrossRef]
- Kamal Hussien, M.; Sabbah, A.; Qorbani, M.; Hammad Elsayed, M.; Raghunath, P.; Lin, T.-Y.; Quadir, S.; Wang, H.-Y.; Wu, H.-L.; Tzou, D.-L.M.; et al. Metal-free four-in-one modification of g-C3N4 for superior photocatalytic CO2 reduction and H2 evolution. Chem. Eng. J. 2022, 430, 132853. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Endo-Kimura, M.; Wang, K.; Ohtani, B.; Kowalska, E. Development of Sulfur-Doped Graphitic Carbon Nitride for Hydrogen Evolution under Visible-Light Irradiation. Nanomaterials 2023, 13, 62. [Google Scholar] [CrossRef]
- Yu, S.; Li, C.; Wu, H.; Wang, Y.; Wang, L.; Dong, H.; Han, Z. Ultrathin Mesoporous Carbon Nitride Nanosheets Prepared Through a One-Pot Approach towards Enhanced Photocatalytic Activity. Energy Technol. 2020, 8, 2000719. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; Zhang, H.; Li, Z.; Wen, Y. One-pot synthesis of porous g-C3N4 nanosheets with enhanced photocatalytic activity under visible light. Diam. Relat. Mater. 2021, 116, 108416. [Google Scholar] [CrossRef]
- He, F.; Chen, G.; Yu, Y.; Zhou, Y.; Zheng, Y.; Hao, S. The sulfur-bubble template-mediated synthesis of uniform porous g-C3N4 with superior photocatalytic performance. Chem. Commun. 2015, 51, 425–427. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, G.; Wang, X. Synthesis of Carbon Nitride Semiconductors in Sulfur Flux for Water Photoredox Catalysis. ACS Catal. 2012, 2, 940–948. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, Y.; Zhao, Z. Porous defect-modified graphitic carbon nitride via a facile one-step approach with significantly enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Catal. B 2018, 226, 1–9. [Google Scholar] [CrossRef]
- Wu, X.; Ma, H.; Zhong, W.; Fan, J.; Yu, H. Porous crystalline g-C3N4: Bifunctional NaHCO3 template-mediated synthesis and improved photocatalytic H2-evolution rate. Appl. Catal. B 2020, 271, 118899. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, Z.; Tang, C.; Zhou, Y.; Zeng, L.; Huang, L. Enhancement of photocatalytic hydrogen evolution activity of porous oxygen doped g-C3N4 with nitrogen defects induced by changing electron transition. Appl. Catal. B 2019, 240, 30–38. [Google Scholar] [CrossRef]
- Huang, W.; Johnston-Peck, A.C.; Wolter, T.; Yang, W.-C.D.; Xu, L.; Oh, J.; Reeves, B.A.; Zhou, C.; Holtz, M.E.; Herzing, A.A.; et al. Steam-created grain boundaries for methane C-H activation in palladium catalysts. Science 2021, 373, 1518–1523. [Google Scholar] [CrossRef]
- Long, B.; Yan, G.; He, H.; Meng, S. Porous and Few-Layer Carbon Nitride Nanosheets via Surface Steam Etching for Enhanced Photodegradation Activity. ACS Appl. Nano Mater. 2022, 5, 7798–7810. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Song, C.; Zhou, S.; Yang, F.; Kong, Y. Engineering carbon-defects on ultrathin g-C3N4 allows one-pot output and dramatically boosts photoredox catalytic activity. Appl. Catal. B 2021, 295, 120272. [Google Scholar] [CrossRef]
- Tay, Q.; Kanhere, P.; Ng, C.F.; Chen, S.; Chakraborty, S.; Huan, A.C.H.; Sum, T.C.; Ahuja, R.; Chen, Z. Defect Engineered g-C3N4 for Efficient Visible Light Photocatalytic Hydrogen Production. Chem. Mater. 2015, 27, 4930–4933. [Google Scholar] [CrossRef]
- Wang, X.; Meng, J.; Zhang, X.; Liu, Y.; Ren, M.; Yang, Y.; Guo, Y. Controllable Approach to Carbon-Deficient and Oxygen-Doped Graphitic Carbon Nitride: Robust Photocatalyst Against Recalcitrant Organic Pollutants and the Mechanism Insight. Adv. Funct. Mater. 2021, 31, 2010763. [Google Scholar] [CrossRef]
- Wang, X.; Xia, Y.; Wang, H.; Jiao, X.; Chen, D. Etching-induced highly porous polymeric carbon nitride with enhanced photocatalytic hydrogen evolution. Chem. Commun. 2021, 57, 4138–4141. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Yuan, Y.; Li, L.; Zhang, M.; Zhou, C.; Lin, Z. A Rapid Microwave-Assisted Thermolysis Route to Highly Crystalline Carbon Nitrides for Efficient Hydrogen Generation. Angew. Chem. Int. Ed. 2016, 55, 14693–14697. [Google Scholar] [CrossRef]
- Shi, L.; Yang, L.; Zhou, W.; Liu, Y.; Yin, L.; Hai, X.; Song, H.; Ye, J. Photoassisted Construction of Holey Defective g-C3N4 Photocatalysts for Efficient Visible-Light-Driven H2O2 Production. Small 2018, 14, 1703142. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yue, X.; Zhang, D.; Fan, J.; Xiang, Q. Targeted regulation of exciton dissociation in graphitic carbon nitride by vacancy modification for efficient photocatalytic CO2 reduction. Appl. Catal. B 2021, 292, 120179. [Google Scholar] [CrossRef]
- Zhang, G.; Li, G.; Lan, Z.-A.; Lin, L.; Savateev, A.; Heil, T.; Zafeiratos, S.; Wang, X.; Antonietti, M. Optimizing Optical Absorption, Exciton Dissociation, and Charge Transfer of a Polymeric Carbon Nitride with Ultrahigh Solar Hydrogen Production Activity. Angew. Chem. Int. Ed. 2017, 56, 13445–13449. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, B.; He, H.; Yu, Y.; Cai, W.; Gu, Q.; Yang, J.; Meng, S. Bifunctional Hot Water Vapor Template-Mediated Synthesis of Nanostructured Polymeric Carbon Nitride for Efficient Hydrogen Evolution. Molecules 2023, 28, 4862. https://doi.org/10.3390/molecules28124862
Long B, He H, Yu Y, Cai W, Gu Q, Yang J, Meng S. Bifunctional Hot Water Vapor Template-Mediated Synthesis of Nanostructured Polymeric Carbon Nitride for Efficient Hydrogen Evolution. Molecules. 2023; 28(12):4862. https://doi.org/10.3390/molecules28124862
Chicago/Turabian StyleLong, Baihua, Hongmei He, Yang Yu, Wenwen Cai, Quan Gu, Jing Yang, and Sugang Meng. 2023. "Bifunctional Hot Water Vapor Template-Mediated Synthesis of Nanostructured Polymeric Carbon Nitride for Efficient Hydrogen Evolution" Molecules 28, no. 12: 4862. https://doi.org/10.3390/molecules28124862