A Comparative Evaluation of the Photosensitizing Efficiency of Porphyrins, Chlorins and Isobacteriochlorins toward Melanoma Cancer Cells
Abstract
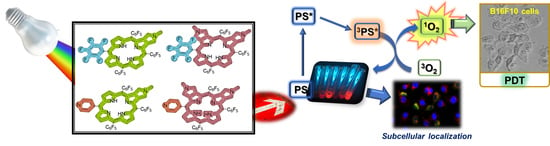
:1. Introduction
2. Results and Discussion
2.1. Photosensitizers: Synthesis and Characterization
2.2. PDT Assays
3. Materials and Methods
3.1. Generalities
3.2. Synthesis of the Photosensitizers (PS)
3.3. Cell Culture
3.4. Cell Viability Assay
3.5. Statistical Analysis
3.6. Fluorescence Studies
3.7. Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Swavey, S.; Tran, M. Porphyrin and Phthalocyanine Photosensitizers as PDT Agents: A New Modality for the Treatment of Melanoma. In Recent Advances in the Biology, Therapy and Management of Melanoma; Davids, L.M., Ed.; InTech: Rijejka, Croatia, 2013. [Google Scholar]
- Huang, Y.Y.; Vecchio, D.; Avci, P.; Yin, R.; Garcia-Diaz, M.; Hamblin, M.R. Melanoma resistance to photodynamic therapy: New insights. Biol. Chem. 2013, 394, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.T.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Ferreira, V.F.; Juarranz, A.; Cavaleiro, J.A.S.; Sanz-Rodríguez, F. Photodynamic effect of glycochlorin conjugates in human cancer epithelial cells. RSC Adv. 2015, 5, 33496–33502. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. JNCI-J. Natl. Cancer I. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Korolchuk, A.M.; Zolottsev, V.A.; Misharin, A.Y. Conjugates of Tetrapyrrolic Macrocycles as Potential Anticancer Target-Oriented Photosensitizers. Top. Curr. Chem. 2023, 381, 10. [Google Scholar] [CrossRef]
- Pan, L.; Ma, Y.; Wu, X.; Cai, H.; Qin, F.; Wu, H.; Li, C.Y.; Jia, Z. A Brief Introduction to Porphyrin Compounds used in Tumor Imaging and Therapies. Mini Rev. Med. Chem. 2021, 21, 1303–1313. [Google Scholar] [CrossRef]
- Zheng, B.-D.; Ye, J.; Zhang, X.-Q.; Zhang, N.; Xiao, M.-T. Recent advances in supramolecular activatable phthalocyanine-based photosensitizers for anti-cancer therapy. Coord. Chem. Rev. 2021, 447, 214155. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Dandash, F.; Leger, D.Y.; Diab-Assaf, M.; Sol, V.; Liagre, B. Porphyrin/Chlorin Derivatives as Promising Molecules for Therapy of Colorectal Cancer. Molecules 2021, 26, 7268. [Google Scholar] [CrossRef]
- Pucelik, B.; Sulek, A.; Drozd, A.; Stochel, G.; Pereira, M.M.; Pinto, S.M.A.; Arnaut, L.G.; Dabrowski, J.M. Enhanced Cellular Uptake and Photodynamic Effect with Amphiphilic Fluorinated Porphyrins: The Role of Sulfoester Groups and the Nature of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueira, F.; Pereira, P.M.R.; Silva, S.; Cavaleiro, J.A.S.; Tomé, J.P.C. Porphyrins and Phthalocyanines Decorated with Dendrimers: Synthesis and Biomedical Applications. Curr. Org. Synth. 2014, 11, 110–126. [Google Scholar] [CrossRef]
- Simplicio, F.I.; Maionchi, F.n.; Hioka, N. Terapia fotodinâmica: Aspectos farmacológicos, aplicações e avanços recentes no desenvolvimento de medicamentos. Quim. Nova 2002, 25, 801–807. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Arnaut, L.G.; Pereira, M.M.; Monteiro, C.J.; Urbanska, K.; Simoes, S.; Stochel, G. New halogenated water-soluble chlorin and bacteriochlorin as photostable PDT sensitizers: Synthesis, spectroscopy, photophysics, and in vitro photosensitizing efficacy. ChemMedChem 2010, 5, 1770–1780. [Google Scholar] [CrossRef]
- Habermeyer, B.; Guilard, R. Some activities of PorphyChem illustrated by the applications of porphyrinoids in PDT, PIT and PDI. Photochem. Photobiol. Sci. 2018, 17, 1675–1690. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Goslinski, T.; Piskorz, J. Fluorinated porphyrinoids and their biomedical applications. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 304–321. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef]
- Hao, E.; Friso, E.; Miotto, G.; Jori, G.; Soncin, M.; Fabris, C.; Sibrian-Vazquez, M.; Vicente, M.G. Synthesis and biological investigations of tetrakis(p-carboranylthio-tetrafluorophenyl)chlorin (TPFC). Org. Biomol. Chem. 2008, 6, 3732–3740. [Google Scholar] [CrossRef]
- Kralova, J.; Synytsya, A.; Pouckova, P.; Koc, M.; Dvorak, M.; Kral, V. Novel Porphyrin Conjugates with a Potent Photodynamic Antitumor Effect: Differential Efficacy of Mono- and Bis-β-cyclodextrin Derivatives In Vifro and In Vivo. Photochem. Photobiol. 2006, 82, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Králová, J.; Bříza, T.; Moserová, I.; Dolenský, B.; Vašek, P.; Poučková, P.; Kejík, Z.; Kaplánek, R.; Martásek, P.; Dvořák, M.; et al. Glycol Porphyrin Derivatives as Potent Photodynamic Inducers of Apoptosis in Tumor Cells. J. Med. Chem. 2008, 51, 5964–5973. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.; Silva, S.; Ramalho, J.S.; Gomes, C.M.; Girão, H.; Cavaleiro, J.A.; Ribeiro, C.A.; Tomé, J.P.; Fernandes, R. The role of galectin-1 in in vitro and in vivo photodynamic therapy with a galactodendritic porphyrin. Eur. J. Cancer 2016, 68, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.A.M.; Laranjo, M.; Pina, J.; Oliveira, A.S.R.; Ferreira, J.D.; Sanchez-Sanchez, C.; Casalta-Lopes, J.; Goncalves, A.C.; Sarmento-Ribeiro, A.B.; Pineiro, M.; et al. Advances on photodynamic therapy of melanoma through novel ring-fused 5,15-diphenylchlorins. Eur. J. Med. Chem. 2018, 146, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Mota, M.; Fernandes, C.; Sequeira, D.; Palma, P.; Caramelo, F.; Neves, M.; Faustino, M.A.F.; Goncalves, T.; Santos, J.M. Is the chlorophyll derivative Zn(II)e(6)Me a good photosensitizer to be used in root canal disinfection? Photodiagnosis Photodyn. Ther. 2018, 22, 205–211. [Google Scholar] [CrossRef]
- Pandey, R.K.; Zheng, G. Applications: Past, Present and Future. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2000; Volume 6. [Google Scholar]
- Stockert, J.C.; Cañete, M.; Juarranz, A.; Villanueva, A.; Horobin, R.W.; Borrell, J.I.; Teixidó, J.; Nonell, S. Porphycenes: Facts and prospects in photodynamic therapy of cancer. Curr. Med. Chem. 2007, 14, 997–1026. [Google Scholar] [CrossRef]
- Senge, M.O.; Sergeeva, N.N.; Hale, K.J. Classic highlights in porphyrin and porphyrinoid total synthesis and biosynthesis. Chem. Soc. Rev. 2021, 50, 4730–4789. [Google Scholar] [CrossRef]
- Koifman, O.I.; Stuzhin, P.A.; Travkin, V.V.; Pakhomov, G.L. Chlorophylls in thin-film photovoltaic cells, a critical review. RSC Adv. 2021, 11, 15131–15152. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Monteiro, C.J.P.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Synthesis of chlorins and bacteriochlorins from cycloaddition reactions with porphyrins. ARKIVOC 2022, 2022, 54–98. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Ramos, L.; Mesquita, M.; Biazzotto, J.C.; Moura, N.M.M.; Mendes, R.F.; Almeida Paz, F.A.; Tomé, A.C.; Cavaleiro, J.A.S.; Simões, M.M.Q.; et al. Comparison of the Photodynamic Action of Porphyrin, Chlorin, and Isobacteriochlorin Derivatives toward a Melanotic Cell Line. ACS Appl. Bio Mater. 2021, 4, 4925–4935. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Ferreira, A.R.; Neves, M.d.G.P.M.S.; Ribeiro, D.; Fardilha, M.; Faustino, M.A.F. Photodynamic therapy of prostate cancer using porphyrinic formulations. J. Photochem. Photobiol. B 2021, 223, 112301. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, A.; Thompson, S.; Tomé, J.P.; Zhu, X.; Samaroo, D.; Vinodu, M.; Gao, R.; Drain, C.M. Synthesis and photophysical properties of thioglycosylated chlorins, isobacteriochlorins, and bacteriochlorins for bioimaging and diagnostics. Bioconjug. Chem. 2010, 21, 2136–2146. [Google Scholar] [CrossRef] [Green Version]
- Samaroo, D.; Zahran, M.; Wills, A.C.; Guevara, J.; Tatonetti, A. In vitro interaction and computational studies of glycosylated photosensitizers with plasma proteins. J. Porphyrins Phthalocyanines 2019, 23, 437–452. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Pires, S.M.G.; Ribeiro, M.A.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Schreiner, W.H.; Wypych, F.; Cavaleiro, J.A.S.; Nakagaki, S. Manganese chlorins immobilized on silica as oxidation reaction catalysts. J. Colloid Interface Sci. 2015, 450, 339–352. [Google Scholar] [CrossRef]
- Maestrin, A.P.J.; Ribeiro, A.O.; Tedesco, A.C.; Neri, C.R.; Vinhado, F.S.; Serra, O.A.; Martins, P.R.; Iamamoto, Y.; Silva, A.M.G.; Tomé, A.C.; et al. A novel chlorin derivative of meso-tris(pentafluorophenyl)-4-pyridylporphyrin: Synthesis, photophysics and photochemical properties. J. Braz. Chem. Soc. 2004, 15, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A.; Hackbarth, S.; Roder, B.; Faustino, M.A.F. Photodynamic inactivation of bioluminescent Escherichia coli by neutral and cationic pyrrolidine-fused chlorins and isobacteriochlorins. Bioorg. Med. Chem. Lett. 2014, 24, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S. meso-tetraarylporphyrins as dipolarophiles in 1,3-dipolar cycloaddition reactions. Chem. Commun. 1999, 1767–1768. [Google Scholar] [CrossRef]
- Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S. 1,3-dipolar cycloaddition reactions of porphyrins with azomethine ylides. J. Org. Chem. 2005, 70, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Gouterman, M. Spectra of Porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Uttamlal, M.; Sheila Holmes-Smith, A. The excitation wavelength dependent fluorescence of porphyrins. Chem. Phys. Lett. 2008, 454, 223–228. [Google Scholar] [CrossRef]
- Hyland, M.A.; Morton, M.D.; Brückner, C. meso-Tetrakis(pentafluorophenyl)porphyrin-Derived Chromene-Annulated Chlorins. J. Org. Chem. 2012, 77, 3038–3048. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Sniechowska, J.; Ziental, D.; Dlugaszewska, J.; Potrzebowski, M.J. Chlorins with (trifluoromethyl)phenyl substituents—Synthesis, lipid formulation and photodynamic activity against bacteria. Dye. Pigm. 2019, 160, 292–300. [Google Scholar] [CrossRef]
- Seybold, P.G.; Gouterman, M.; Callis, J. Calorimetric, photometric and lifetime determinations of fluorescence yields of fluorescein dyes. Photochem. Photobiol. 1969, 9, 229–242. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Comprehensive review of photophysical parameters (ε, Φf, τs) of tetraphenylporphyrin (H2TPP) and zinc tetraphenylporphyrin (ZnTPP)—Critical benchmark molecules in photochemistry and photosynthesis. J. Photochem. Photobiol. C Photochem. Rev. 2021, 46, 100401. [Google Scholar] [CrossRef]
- Jiménez-Osés, G.; García, J.I.; Silva, A.M.G.; Santos, A.R.N.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Mechanistic insights on the site selectivity in successive 1,3-dipolar cycloadditions to meso-tetraarylporphyrins. Tetrahedron 2008, 64, 7937–7943. [Google Scholar] [CrossRef]
- Gentemann, S.; Medforth, C.J.; Forsyth, T.P.; Nurco, D.J.; Smith, K.M.; Fajer, J.; Holten, D. Photophysical Properties of Conformationally Distorted Metal-Free Porphyrins. Investigation into the Deactivation Mechanisms of the Lowest Excited Singlet State. J. Am. Chem. Soc. 1994, 116, 7363–7368. [Google Scholar] [CrossRef]
- Cavaleiro, J.A.S.; Görner, H.; Lacerda, P.S.S.; MacDonald, J.G.; Mark, G.; Neves, M.G.P.M.S.; Nohr, R.S.; Schuchmann, H.-P.; von Sonntag, C.; Tomé, A.C. Singlet oxygen formation and photostability of meso-tetraarylporphyrin derivatives and their copper complexes. J. Photochem. Photobiol. A Chem. 2001, 144, 131–140. [Google Scholar] [CrossRef]
- Souza, T.G.B.d.; Vivas, M.G.; Mendonça, C.R.; Plunkett, S.; Filatov, M.A.; Senge, M.O.; Boni, L.D. Studying the intersystem crossing rate and triplet quantum yield of meso-substituted porphyrins by means of pulse train fluorescence technique. J. Porphyrins Phthalocyanines 2016, 20, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Spiller, W.; Kliesch, H.; Wöhrle, D.; Hackbarth, S.; Röder, B.; Schnurpfeil, G. Singlet Oxygen Quantum Yields of Different Photosensitizers in Polar Solvents and Micellar Solutions. J. Porphyrins Phthalocyanines 1998, 02, 145–158. [Google Scholar] [CrossRef]
- Nifiatis, F.; Athas, J.C.; Gunaratne, K.D.D.; Gurung, Y.; Monette, K.M.; Shivokevich, P.J. Substituent Effects of Porphyrin on Singlet Oxygen Generation Quantum Yields. Open Spectrosc. J. 2011, 5, 1–12. [Google Scholar] [CrossRef]
- Topkaya, D.; Arnoux, P.; Dumoulin, F. Modulation of singlet oxygen generation and amphiphilic properties of trihydroxylated monohalogenated porphyrins. J. Porphyrins Phthalocyanines 2015, 19, 1081–1087. [Google Scholar] [CrossRef]
- Le Guern, F.; Ouk, T.S.; Yerzhan, I.; Nurlykyz, Y.; Arnoux, P.; Frochot, C.; Leroy-Lhez, S.; Sol, V. Photophysical and Bactericidal Properties of Pyridinium and Imidazolium Porphyrins for Photodynamic Antimicrobial Chemotherapy. Molecules 2021, 26, 1122. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Stornetta, A.; Zimmermann, M.; Cimino, G.D.; Henderson, P.T.; Sturla, S.J. DNA Adducts from Anticancer Drugs as Candidate Predictive Markers for Precision Medicine. Chem. Res. Toxicol. 2017, 30, 388–409. [Google Scholar] [CrossRef]
- Salomao, G.H.A.; Fernandes, A.U.; Baptista, M.S.; Tardivo, J.P.; Gianssante, S.; Veridiano, J.M.; Toledo, O.M.S.; Petri, G.; Christofolini, D.M.; Correa, J.A. A new Chlorin formulation promotes efficient photodynamic action in choriocapillaris of rabbit’s eyes. Bioorg. Med. Chem. Lett. 2018, 28, 1870–1873. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Marek, G.; Serpa, C.; Szurko, A.; Widel, M.; Sochanik, A.; Snietura, M.; Kus, P.; Nunes, R.M.; Arnaut, L.G.; Ratuszna, A. Spectroscopic properties and photodynamic effects of new lipophilic porphyrin derivatives: Efficacy, localisation and cell death pathways. J. Photochem. Photobiol. B Biol. 2006, 84, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisland, S.K.; Lilge, L.; Lin, A.; Rusnov, R.; Wilson, B.C. Metronomic photodynamic therapy as a new paradigm for photodynamic therapy: Rationale and preclinical evaluation of technical feasibility for treating malignant brain tumors. Photochem. Photobiol. 2004, 80, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.A.D.F.; Prandini, J.A.; Biazzotto, J.C.; Tomé, J.P.C.; da Silva, R.S.; Lourenço, L.M.O. The Surprisingly Positive Effect of Zinc-Phthalocyanines With High Photodynamic Therapy Efficacy of Melanoma Cancer. Front. Chem. 2022, 10, 825716. [Google Scholar] [CrossRef]
- Gümrükçü, G.; Karaoğlan, G.K.; Erdoğmuş, A.; Gül, A.; Avcıata, U. Photophysical, Photochemical, and BQ Quenching Properties of Zinc Phthalocyanines with Fused or Interrupted Extended Conjugation. J. Chem. 2014, 2014, 435834. [Google Scholar] [CrossRef] [Green Version]
- Mazur, L.M.; Roland, T.; Leroy-Lhez, S.; Sol, V.; Samoc, M.; Samuel, I.D.W.; Matczyszyn, K. Efficient Singlet Oxygen Photogeneration by Zinc Porphyrin Dimers upon One- and Two-Photon Excitation. J. Phys. Chem. B 2019, 123, 4271–4277. [Google Scholar] [CrossRef] [Green Version]





| Compound | Soret Band λmax (nm) | Q Bands λmax (nm) | a λemission (nm) | b ΦF | c τ1 (ns) | c τ2 (ns) | c τT (μs) | d ΦΔ | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Por1 | 410 | 504 | 539 | 582 | 638 | 638/698 | 0.01 | 10.0 (100) | - | 0.98 | 0.55 |
| Chl1 | 405 | 505 | 540 | 595 | 653 | 654 | 0.15 | 6.02 (100) | - | 0.51 | 0.42 |
| Iso1 | 380 | 510 | 548 | 586 | 648 | 600/654 | 0.13 | 5.40 (97) | 1.20 (3.0) | 0.50 | 0.31 |
| Por2 | 412 | 506 | 538 | 582 | 635 | 638/700 | 0.06 | 11.1 (100) | - | 0.76 | 0.65 |
| Chl2 | 406 | 504 | 532 | 595 | 649 | 649 | 0.16 | 6.90 (100) | - | 0.74 | 0.81 |
| Iso2 | 386 | 506 | 540 | 580 | 660 | 638/700 | 0.21 | 6.39 (23) | 1.58 (77) | 0.63 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, K.A.D.F.; Moura, N.M.M.; Simões, M.M.Q.; Mesquita, M.M.Q.; Ramos, L.C.B.; Biazzotto, J.C.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; da Silva, R.S. A Comparative Evaluation of the Photosensitizing Efficiency of Porphyrins, Chlorins and Isobacteriochlorins toward Melanoma Cancer Cells. Molecules 2023, 28, 4716. https://doi.org/10.3390/molecules28124716
Castro KADF, Moura NMM, Simões MMQ, Mesquita MMQ, Ramos LCB, Biazzotto JC, Cavaleiro JAS, Faustino MAF, Neves MGPMS, da Silva RS. A Comparative Evaluation of the Photosensitizing Efficiency of Porphyrins, Chlorins and Isobacteriochlorins toward Melanoma Cancer Cells. Molecules. 2023; 28(12):4716. https://doi.org/10.3390/molecules28124716
Chicago/Turabian StyleCastro, Kelly A. D. F., Nuno M. M. Moura, Mário M. Q. Simões, Mariana M. Q. Mesquita, Loyanne C. B. Ramos, Juliana C. Biazzotto, José A. S. Cavaleiro, M. Amparo F. Faustino, Maria Graça P. M. S. Neves, and Roberto S. da Silva. 2023. "A Comparative Evaluation of the Photosensitizing Efficiency of Porphyrins, Chlorins and Isobacteriochlorins toward Melanoma Cancer Cells" Molecules 28, no. 12: 4716. https://doi.org/10.3390/molecules28124716