Occurrence and Characteristics of Microplastics in Wild and Farmed Shrimps Collected from Cau Hai Lagoon, Central Vietnam
Abstract
:1. Introduction
2. Results
2.1. Abundance of Microplastics
2.2. Morphological Characteristics of Microplastics in Shrimps
2.3. Identification of Microplastics in Shrimps with ATR-FTIR
3. Discussion
3.1. Abundance of MPs in Shrimp Samples
3.2. Morphological Characteristics of MPs
3.3. Polymer Composition of MPs
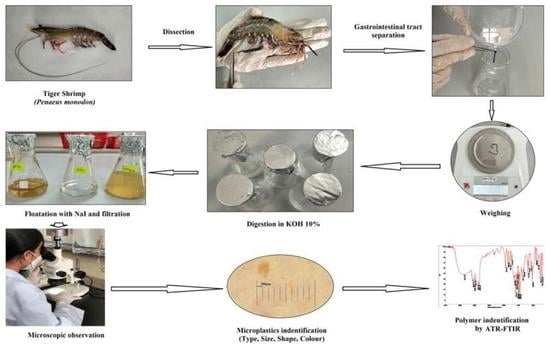
4. Materials and Method
4.1. Chemicals
4.2. Sample Collection and Processing
4.3. Samples Preparation, Digestion, and Observation
4.4. Identification of Microplastics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PlasticEurope. Plastics—The Facts 2016. An Analysis of European Latest Plastics Production, Demand and Waste Data. 2016. Available online: https://www.plasticseurope.org/application/files/4315/1310/4805/plastic-the-fact-2016.pdf (accessed on 7 August 2017).
- Lebreton, L.C.; Van der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from MP to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and MP in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- My, T.T.A.; Dat, N.D.; Hung, N.Q.; Quang, D.T. Preliminary determination of microplastics in bivalves collected from Phu Yen, central Viet Nam. Vietnam. J. Sci. Technol. 2023, 61, 480–490. [Google Scholar]
- Bessa, F.; Barría, P.; Neto, J.M.; Frias, J.P.G.L.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Janssen, C. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Kirkham, M.B. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019, 131, 104937. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Lahens, L.; Strady, E.; Kieu-Le, T.-C.; Dris, R.; Boukerma, K.; Rinnert, E.; Gasperi, J.; Tassin, B. Macroplastic and microplastic contamination assessment of a tropical river (Saigon River, Vietnam) transversed by a developing megacity. Environ. Pollut. 2018, 236, 661–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Hossain, M.S.; Rahman, M.S.; Uddin, M.N.; Sharifuzzaman, S.; Chowdhury, S.R.; Sarker, S.; Chowdhury, M.S.N. Microplastic contamination in Penaeid shrimp from the Northern Bay of Bengal. Chemosphere 2020, 238, 124688. [Google Scholar] [CrossRef]
- Curren, E.; Leaw, C.P.; Lim, P.T.; Leong, S.C.Y. Evidence of Marine Microplastics in Commercially Harvested Seafood. Front. Bioeng. Biotechnol. 2020, 8, 562760. [Google Scholar] [CrossRef]
- Gurjar, U.R.; Xavier, M.; Nayak, B.B.; Ramteke, K.; Deshmukhe, G.; Jaiswar, A.K.; Shukla, S.P. Microplastics in shrimps: A study from the trawling grounds of north eastern part of Arabian Sea. Environ. Sci. Pollut. Res. 2021, 28, 48494–48504. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [Green Version]
- Suman, T.Y.; Jia, P.P.; Li, W.G.; Junaid, M.; Xin, G.Y.; Wang, Y.; Pei, D.S. Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard. Mater. 2020, 400, 123220. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Hollman, P.C.H.; Peters, R.J.B. Potential health impact of environmentally released micro- and nano-plastics in the human food production chain: Experiences from nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, S.; Romano, S.; Turetta, C.; Cu, N.H.; Bellucci, L.G.; Capodaglio, G.; Mugnai, C.; Nhon, D.H.; Frignani, M. Soils and sediments of the Thua Thien-Hue Province (central Vietnam): Recognizing trace element sources and the likely influence of natural events. J. Environ. Monit. 2011, 13, 1383–1392. [Google Scholar] [CrossRef]
- Bour, A.; Avio, C.G.; Gorbi, S.; Regoli, F.; Hylland, K. Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environ. Pollut. 2018, 243, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating microplastics bioaccumulation and biomagnification in seafood from the Persian Gulf: A threat to human health? Food Addit. Contam. Part A 2019, 36, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N.; Thomson, K.T. Microplastics in the edible tissues of shellfishes sold for human consumption. Chemosphere 2021, 264, 128554. [Google Scholar] [CrossRef]
- Nan, B.; Su, L.; Kellar, C.; Craig, N.J.; Keough, M.J.; Pettigrove, V. Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria, Australia. Environ. Pollut. 2020, 259, 113865. [Google Scholar] [CrossRef]
- Valencia-Castañeda, G.; Ruiz-Fernández, A.C.; Frías-Espericueta, M.G.; Rivera-Hernández, J.R.; Green-Ruiz, C.R.; Páez-Osuna, F. Microplastics in the tissues of commercial semi-intensive shrimp pond-farmed Litopenaeus vannamei from the Gulf of California ecoregion. Chemosphere 2022, 297, 134194. [Google Scholar] [CrossRef] [PubMed]
- My, T.T.A.; Dat, N.D.; Long, H.T.; Binh, T.T. Occurrence of microdebris in muscle of round scad (Decapterus maruadsi) collected from Central Vietnam. Environ. Asia 2022, 15, 38–47. [Google Scholar]
- Fang, C.; Zheng, R.; Hong, F.; Jiang, Y.; Chen, J.; Lin, H. Microplastics in three typical benthic species from the Arctic: Occurrence, characteristics, sources, and environmental implications. Environ. Res. 2021, 192, 110326. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tong, C.; Wu, F.; Jiang, S.; Zhang, S. Distribution characteristics of microplastics and corresponding feeding habits of the dominant shrimps in the rivers of Chongming Island. Sci. Total Environ. 2023, 888, 164041. [Google Scholar] [CrossRef] [PubMed]
- Severini, M.F.; Buzzi, N.S.; López, A.F.; Colombo, C.V.; Sartor, G.C.; Rimondino, G.N.; Truchet, D.M. Chemical composition and abundance of microplastics in the muscle of commercial shrimp Pleoticus muelleri at an impacted coastal environment (Southwestern Atlantic). Mar. Pollut. Bull. 2020, 161, 111700. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, W.; Chen, X.; He, Y.; Zhang, X.; Gong, H. A preliminary study of the association between colonization of microorganism on microplastics and intestinal microbiota in shrimp under natural conditions. J. Hazard. Mater. 2021, 408, 124882. [Google Scholar] [CrossRef]
- Wehrheim, C.; Lübken, M.; Stolpe, H.; Wichern, M. Identifying Key Influences on Surface Water Quality in Freshwater Areas of the Vietnamese Mekong Delta from 2018 to 2020. Water 2023, 15, 1295. [Google Scholar] [CrossRef]
- Murphy, C.L. A Comparison of Microplastics in Farmed and Wild Shellfish near Vancouver Island and Potential Implications for Contaminant Transfer to Humans. Ph.D. Thesis, Royal Roads University, Victoria, BC, Canada, 2018. [Google Scholar]
- Yin, J.; Li, J.-Y.; Craig, N.J.; Su, L. Microplastic pollution in wild populations of decapod crustaceans: A review. Chemosphere 2022, 291, 132985. [Google Scholar] [CrossRef]
- Atamanalp, M.; Köktürk, M.; Uçar, A.; Duyar, H.A.; Özdemir, S.; Parlak, V.; Esenbuğa, N.; Alak, G. Microplastics in Tissues (Brain, Gill, Muscle and Gastrointestinal) of Mullus barbatus and Alosa immaculata. Arch. Environ. Contam. Toxicol. 2021, 81, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Lusher, A.L.; Burke, A.; O’connor, I.; Officer, R. Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling. Mar. Pollut. Bull. 2014, 88, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; Martínez-Gómez, C. Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef]
- Compa, M.; Ventero, A.; Iglesias, M.; Deudero, S. Ingestion of microplastics and natural fibers in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean coast. Mar. Pollut. Bull. 2018, 128, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of MP in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Savoca, S.; Capillo, G.; Mancuso, M.; Faggio, C.; Panarello, G.; Crupi, R.; Bonsignore, M.; D’Urso, L.; Compagnini, G.; Neri, F.; et al. Detection of artificial cellulose microfibers in Boops boops from the northern coasts of Sicily (Central Mediterranean). Sci. Total Environ. 2019, 691, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Chinfak, N.; Sompongchaiyakul, P.; Charoenpong, C.; Shi, H.; Yeemin, T.; Zhang, J. Abundance, composition, and fate of microplastics in water, sediment, and shellfish in the Tapi-Phumduang River system and Bandon Bay, Thailand. Sci. Total Environ. 2021, 781, 146700. [Google Scholar] [CrossRef]
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- Macieira, R.M.; Oliveira, L.A.S.; Cardozo-Ferreira, G.C.; Pimentel, C.R.; Andrades, R.; Gasparini, J.L.; Sarti, F.; Chelazzi, D.; Cincinelli, A.; Gomes, L.C.; et al. Microplastic and artificial cellulose microfibers ingestion by reef fishes in the Guarapari Islands, southwestern Atlantic. Mar. Pollut. Bull. 2021, 167, 112371. [Google Scholar] [CrossRef] [PubMed]
- Suaria, G.; Achtypi, A.; Perold, V.; Lee, J.R.; Pierucci, A.; Bornman, T.G.; Aliani, S.; Ryan, P.G. Microfibers in oceanic surface waters: A global characterization. Sci. Adv. 2020, 6, aay8493. [Google Scholar] [CrossRef]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Specie | Type of Sample | Levels of MPs (Items/Individual) | Levels of MPs (Items/g-ww) | Size (μm) | Microplastic Type | Nation | Reference |
|---|---|---|---|---|---|---|---|
| Greasy-back shrimp (Metapenaeus ensis) | Whole | 2.5 ± 0.5 1 | 0.7 ± 0.3 | <100–1000 | Rayon, PET, Polyethylene, Polyamide, Polystyrene, Polyacrylic | Cau Hai Lagoon, Central Vietnam | This study |
| GT | 0.9 ± 0.2 | 2.1 ± 0.3 | <100–1000 | ||||
| Tissue | 1.0 ± 0.8 | 0.5 ± 0.4 | <100–250 | ||||
| Green tiger shrimp (Penaeus semisulcatus) | Whole | 2.3 ± 0.7 1 | 0.6 ± 0.2 | <100–1000 | Rayon, PET, Polyethylene, Polyamide, Polystyrene, Polyacrylic | ||
| GT | 1.4 ± 0.3 * | 3.2 ± 0.7 | <100–500 | ||||
| Tissue | 0.9 ± 0.8 * | 0.3 ± 0.3 | <100–500 | ||||
| White-leg shrimp (Litopenaeus vannamei) | Whole | 8.6 ± 3.5 2 | 1.1 ± 0.4 | <100–1000 | Rayon, PET, Polyethylene, Polyamide | ||
| GT | 6.3 ± 1.4 ** | 104 ± 73 | <100–1000 | ||||
| Tissue | 2.3 ± 0.4 ** | 0.3 ± 0.1 | <100–500 | ||||
| Giant tiger shrimp (Penaeus monodon) | Whole | 7.7 ± 3.5 2 | 0.5 ± 0.3 | <100–2000 | Rayon, PET, Polyethylene, Polyamide | ||
| GT | 5.4 ± 1.3 *** | 28.3 ± 5.7 | <100–1000 | ||||
| Tissue | 2.3 ± 0.7 *** | 0.2 ± 0.1 | <100–500 | ||||
| Crangon crangon | Whole body | 1.23 ± 0.99 | 0.68 ± 0.55 | 200–1000 | Fibers | Southern North Sea | Devriese et al. [15] |
| Crangon allmanni | Whole body | 1–3 | - | - | Fragments and films of polyethylene and polyacrylic | Jeløya, Norway | Bour et al. [23] |
| Penaeus semisulcatus | Muscle | 0.36 | - | 50–5000 μm fibers and <50 μm fragments | Fibers and fragments | Persian Gulf | Akhbarizadeh et al. [24] |
| Fenneropenaeus indicus | Whole body | 0.04 ± 0.07 | - | 157–2785 | Fibers, fragments and films of polyethylene, polypropylene and polyamide | Kochi, India | Daniel et al. [25] |
| Paratya australiensis | - | 24 ± 31 | - | <1–2 mm | Fibers of rayon and polyester | Northern central Victoria, Australia | Nan et al. [26] |
| Penaeus monodon | GT | 6.6 ± 2 | 3.40 ± 1.23 | 250–500 | Fibers, particles and fragments of polyamide 6 and rayon | Northern Bay of Bengal, Bangladesh | Hossain et al. [16] |
| Metapenaeus monoceros | 7.8 ± 2 | 3.87 ± 1.05 | 1000–5000 | ||||
| Litopenaeus vannamei, Pleoticus muelleri, Fenneropenaeus indicus | GT | 21.0 ± 4.0 | - | - | Films, fibers, fragments and spheres | Singapore | Curren et al. [17] |
| Metapenaeus monoceros, Parapeneopsis stylifera, Penaeus indicus | GT | 6.78 ± 2.80 | 70.32 ± 34.67 | 100–250 | Fibers, fragments, beads, pellets and films of polyethylene, polypropylene, polyethylene terephthalate, polyester and polyamide | Arabian Sea | Gurjar et al. [18] |
| Litopenaeus vannamei | Whole | 18.5 ± 1.2 | 1.06 | <2000 | Northwestern Mexico | Valencia-Castañeda et al. [27] | |
| GT | 7.6 ± 0.6 | 261.7 ± 84.5 | |||||
| Gills | 6.3 ± 0.9 | 13.1 ± 1.8 | |||||
| Exoskeleton | 4.3 ± 0.9 | 2.6 ± 0.6 |
| Description | Number of MPs | Percentage (%) | ||||
|---|---|---|---|---|---|---|
| Greasy-Back Metapenaeus ensis | Green Tiger Penaeus semisulcatus | White-Leg Litopenaeus vannamei | Giant Tiger Penaeus monodon | Total Items | ||
| Total selected items | 31 | 25 | 24 | 23 | 105 | 100% |
| Rayon | 18 | 18 | 14 | 15 | 65 | 61.9% |
| PET | 2 | - | 3 | 2 | 7 | 6.7% |
| Polyethylene | 1 | 1 | 2 | 2 | 6 | 5.7% |
| Polyamide | 2 | 2 | 3 | 4 | 11 | 10.5% |
| Polystyrene | 3 | 1 | - | - | 4 | 3.8% |
| Polyacrylic | 3 | 2 | - | - | 5 | 4.8% |
| Unidentified | 2 | 1 | 2 | 2 | 7 | 6.7% |
| Common Name | Species | Habitat | Number of Sample | Shell Length (cm) | Shell Width (cm) | Shell Weight (g/Individual) | Soft Tissue Weight (g/Individual) |
|---|---|---|---|---|---|---|---|
| Greasy-back shrimp | Metapenaeus ensis | Wild | 30 | 8.2 ± 0.3 | 2.0 ± 0.1 | 3.6 ± 0.6 | 2.6 ± 0.4 |
| Green tiger shrimp | Penaeus semisulcatus | Wild | 30 | 10.4 ± 0.7 | 1.4 ± 0.3 | 8.2 ± 1.2 | 4.4 ± 1.0 |
| White-leg shrimp | Litopenaeus vannamei | Farmed | 30 | 13.8 ± 0.8 | 3.0 ± 0.1 | 14.4 ± 2.8 | 8.1 ± 0.8 |
| Giant tiger shrimp | Penaeus monodon | Farmed | 30 | 17.1 ± 1.0 | 3.0 ± 0.1 | 32.4 ± 3.1 | 14.9 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
My, T.T.A.; Dat, N.D.; Hung, N.Q. Occurrence and Characteristics of Microplastics in Wild and Farmed Shrimps Collected from Cau Hai Lagoon, Central Vietnam. Molecules 2023, 28, 4634. https://doi.org/10.3390/molecules28124634
My TTA, Dat ND, Hung NQ. Occurrence and Characteristics of Microplastics in Wild and Farmed Shrimps Collected from Cau Hai Lagoon, Central Vietnam. Molecules. 2023; 28(12):4634. https://doi.org/10.3390/molecules28124634
Chicago/Turabian StyleMy, Tran Thi Ai, Nguyen Duy Dat, and Nguyen Quoc Hung. 2023. "Occurrence and Characteristics of Microplastics in Wild and Farmed Shrimps Collected from Cau Hai Lagoon, Central Vietnam" Molecules 28, no. 12: 4634. https://doi.org/10.3390/molecules28124634