A Descriptive Review of the Action Mechanisms of Berberine, Quercetin and Silymarin on Insulin Resistance/Hyperinsulinemia and Cardiovascular Prevention
Abstract
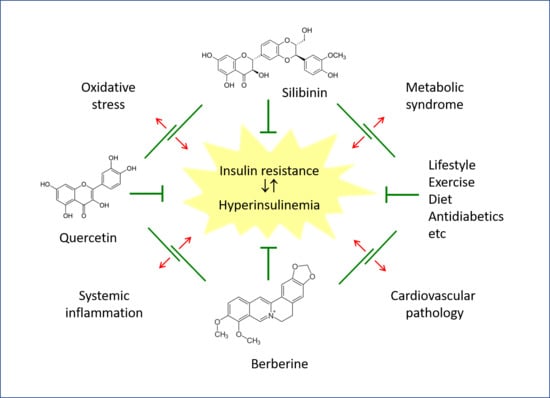
:1. Introduction
2. Insulin Resistance and Hyperinsulinemia: Pathophysiological Mechanisms of Cardiovascular Damage
2.1. Insulin Resistance Mechanisms
2.2. Hyperinsulinaemia as a Cardiovascular Risk Factor
3. Effects of Berberine on Insulin Resistance/Hyperinsulinemia and Cardiovascular Changes
3.1. Effects of Berberine on Glucose Metabolism
3.2. Effects of Berberine on the Cardiovascular System
4. Quercetin as a Modulator of Insulin Resistance
4.1. Antioxidant Action and Inhibition of NADPH Oxidase
4.2. Regulation of Cell Signaling Pathways
4.3. Regulation of Inflammation Associated with Metabolic Disorders
5. Effects of Silymarin on Insulin Resistance/Hyperinsulinemia and Cardiovascular Changes
5.1. Molecular Mechanisms of Silimarin Action on Metabolism
5.2. Clinical Studies of Silymarin for Insulin Resistance
5.3. Effects of Silymarin on the Cardiovascular System
6. Indirect Effects Related to the Microbiota
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonetti, G.; Ugenti, R.; Casciello, M.; Acquaviva, S.; Agrimi, U.; Alario, M.; Alessandrelli, M.; Alfonsi, V.; Aloi, R.; Aloisi, F.; et al. Relazione Sullo Stato Sanitario del Paese 2012–2013. Malattie Cardio-Cerebrovascolari; Ministero della Salute-Presidenza Italiana del Consiglio EU 2014: Roma, Italy, 2014; pp. 72–76. [Google Scholar]
- Istituto Superiore di Sanità. Le Statistiche Delle Malattie Cardiovascolari in Europa per il 2008; Istituto Superiore di Sanità-EpiCentro: Roma, Italy, 2008. [Google Scholar]
- Adeva-Andany, M.M.; Martinez-Rodriguez, J.; Gonzalez-Lucan, M.; Fernandez-Fernandez, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. 2019, 13, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Balkau, B.; Eschwege, E. Insulin resistance: An independent risk factor for cardiovascular disease? Diabetes Obes. Metab. 1999, 1 (Suppl. 1), S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, V.; Salazar, J.; Martinez, M.S.; Chavez-Castillo, M.; Olivar, L.C.; Calvo, M.J.; Palmar, J.; Bautista, J.; Ramos, E.; Cabrera, M.; et al. Prevalence and Associated Factors of Insulin Resistance in Adults from Maracaibo City, Venezuela. Adv. Prev. Med. 2016, 2016, 9405105. [Google Scholar] [CrossRef]
- Zhu, Y.; Sidell, M.A.; Arterburn, D.; Daley, M.F.; Desai, J.; Fitzpatrick, S.L.; Horberg, M.A.; Koebnick, C.; McCormick, E.; Oshiro, C.; et al. Racial/Ethnic Disparities in the Prevalence of Diabetes and Prediabetes by BMI: Patient Outcomes Research To Advance Learning (PORTAL) Multisite Cohort of Adults in the U.S. Diabetes Care 2019, 42, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. 2), S135–S148. [Google Scholar] [CrossRef] [PubMed]
- Affuso, F.; Ruvolo, A.; Micillo, F.; SaccÃ, L.; Fazio, S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Affuso, F.; Mercurio, V.; Ruvolo, A.; Pirozzi, C.; Micillo, F.; Carlomagno, G.; Grieco, F.; Fazio, S. A nutraceutical combination improves insulin sensitivity in patients with metabolic syndrome. World J. Cardiol. 2012, 4, 77–83. [Google Scholar] [CrossRef]
- Affuso, F.; Mercurio, V.; Fazio, V.; Fazio, S. Cardiovascular and metabolic effects of Berberine. World J. Cardiol. 2010, 2, 71–77. [Google Scholar] [CrossRef]
- Mercurio, V.; Carlomagno, G.; Fazio, V.; Fazio, S. Insulin resistance: Is it time for primary prevention? World J. Cardiol. 2012, 4, 1–7. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Marzotto, M.; Conforti, A.; Vella, A.; Ortolani, R.; Bellavite, P. Bimodal action of the flavonoid quercetin on basophil function: An investigation of the putative biochemical targets. Clin. Mol. Allergy 2010, 8, 13. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Conforti, A.; Ortolani, R.; Vella, A.; Marzotto, M.; Bellavite, P. Stimulus-specific regulation of CD63 and CD203c membrane expression in human basophils by the flavonoid quercetin. Int. Immunopharmacol. 2010, 10, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zanini, S.; Marzotto, M.; Giovinazzo, F.; Bassi, C.; Bellavite, P. Effects of dietary components on cancer of the digestive system. Crit. Rev. Food Sci. Nutr. 2015, 55, 1870–1885. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Pennings, N. Insulin Resistance; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tam, C.S.; Xie, W.; Johnson, W.D.; Cefalu, W.T.; Redman, L.M.; Ravussin, E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 2012, 35, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, A.; Esmailzadehha, N.; Oveisi, S.; Ghorbani, A.; Ghanei, L. The threshold value of homeostasis model assessment for insulin resistance in Qazvin Metabolic Diseases Study (QMDS): Assessment of metabolic syndrome. J. Res. Health Sci. 2015, 15, 94–100. [Google Scholar]
- Guerrero-Romero, F.; Simental-Mendia, L.E.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Ramos-Zavala, M.G.; Hernandez-Gonzalez, S.O.; Jacques-Camarena, O.; Rodriguez-Moran, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Qu, H.Q.; Li, Q.; Rentfro, A.R.; Fisher-Hoch, S.P.; McCormick, J.B. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS ONE 2011, 6, e21041. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Kashyap, S.R.; Roman, L.J.; Lamont, J.; Masters, B.S.; Bajaj, M.; Suraamornkul, S.; Belfort, R.; Berria, R.; Kellogg, D.L., Jr.; Liu, Y.; et al. Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J. Clin. Endocrinol. Metab. 2005, 90, 1100–1105. [Google Scholar] [CrossRef]
- Li, Q.; Park, K.; Li, C.; Rask-Madsen, C.; Mima, A.; Qi, W.; Mizutani, K.; Huang, P.; King, G.L. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-beta isoform in the endothelium. Circ. Res. 2013, 113, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Bakris, G.L. Review: Insulin and endothelin: An interplay contributing to hypertension development? J. Clin. Endocrinol. Metab. 2007, 92, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Lasaridis, A.N. Insulin resistance and endothelin: Another pathway for renal injury in patients with the cardiometabolic syndrome? J. Cardiometab. Syndr. 2008, 3, 183–187. [Google Scholar] [CrossRef] [PubMed]
- He, R.J.; Yu, Z.H.; Zhang, R.Y.; Zhang, Z.Y. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol. Sin. 2014, 35, 1227–1246. [Google Scholar] [CrossRef]
- Towler, M.C.; Hardie, D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef]
- Chopra, I.; Li, H.F.; Wang, H.; Webster, K.A. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia 2012, 55, 783–794. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Tang, P.; Tang, Y.; Liu, Y.; He, B.; Shen, X.; Zhang, Z.J.; Qin, D.L.; Tian, J. Quercetin-3-O-alpha-L-arabinopyranosyl-(1-->2)-beta-D-glucopyranoside Isolated from Eucommia ulmoides Leaf Relieves Insulin Resistance in HepG2 Cells via the IRS-1/PI3K/Akt/GSK-3beta Pathway. Biol. Pharm. Bull. 2023, 46, 219–229. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Eseberri, I.; Laurens, C.; Miranda, J.; Louche, K.; Lasa, A.; Moro, C.; Portillo, M.P. Effects of Physiological Doses of Resveratrol and Quercetin on Glucose Metabolism in Primary Myotubes. Int. J. Mol. Sci. 2021, 22, 1384. [Google Scholar] [CrossRef]
- Bachmann, K.N.; Deger, S.M.; Alsouqi, A.; Huang, S.; Xu, M.; Ferguson, J.F.; Su, Y.R.; Niswender, K.D.; Ikizler, T.A.; Wang, T.J. Acute effects of insulin on circulating natriuretic peptide levels in humans. PLoS ONE 2018, 13, e0196869. [Google Scholar] [CrossRef] [PubMed]
- Harith, H.H.; Di Bartolo, B.A.; Cartland, S.P.; Genner, S.; Kavurma, M.M. Insulin promotes vascular smooth muscle cell proliferation and apoptosis via differential regulation of tumor necrosis factor-related apoptosis-inducing ligand. J. Diabetes 2016, 8, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, G.; Pagano, D.; Gnecchi-Ruscone, T.; Bonser, R.S.; Camici, P.G. Insulin resistance in patients with cardiac hypertrophy. Cardiovasc. Res. 1999, 42, 246–253. [Google Scholar] [CrossRef]
- Avruch, J.; Khokhlatchev, A.; Kyriakis, J.M.; Luo, Z.; Tzivion, G.; Vavvas, D.; Zhang, X.F. Ras activation of the Raf kinase: Tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog. Horm. Res. 2001, 56, 127–155. [Google Scholar] [CrossRef] [PubMed]
- Goalstone, M.L.; Leitner, J.W.; Wall, K.; Dolgonos, L.; Rother, K.I.; Accili, D.; Draznin, B. Effect of insulin on farnesyltransferase. Specificity of insulin action and potentiation of nuclear effects of insulin-like growth factor-1, epidermal growth factor, and platelet-derived growth factor. J. Biol. Chem. 1998, 273, 23892–23896. [Google Scholar] [CrossRef]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Zhuge, F.; Ni, Y.; Nagashimada, M.; Nagata, N.; Xu, L.; Mukaida, N.; Kaneko, S.; Ota, T. DPP-4 Inhibition by Linagliptin Attenuates Obesity-Related Inflammation and Insulin Resistance by Regulating M1/M2 Macrophage Polarization. Diabetes 2016, 65, 2966–2979. [Google Scholar] [CrossRef]
- Hattori, S. Omarigliptin decreases inflammation and insulin resistance in a pleiotropic manner in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2020, 12, 24. [Google Scholar] [CrossRef]
- Okura, T.; Fujioka, Y.; Nakamura, R.; Ito, Y.; Kitao, S.; Anno, M.; Matsumoto, K.; Shoji, K.; Okura, H.; Matsuzawa, K.; et al. Dipeptidyl peptidase 4 inhibitor improves insulin resistance in Japanese patients with type 2 diabetes: A single-arm study, a brief report. Diabetol. Metab. Syndr. 2022, 14, 78. [Google Scholar] [CrossRef]
- Lo, C.W.H.; Fei, Y.; Cheung, B.M.Y. Cardiovascular Outcomes in Trials of New Antidiabetic Drug Classes. Card. Fail. Rev. 2021, 7, e04. [Google Scholar] [CrossRef]
- Najjar, S.M.; Caprio, S.; Gastaldelli, A. Insulin Clearance in Health and Disease. Annu. Rev. Physiol. 2023, 85, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Ginsberg, H.N.; Bakris, G.L.; Clark, N.G.; Costa, F.; Eckel, R.; Fonseca, V.; Gerstein, H.C.; Grundy, S.; Nesto, R.W.; et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: A scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007, 115, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Purwaningsih, I.; Maksum, I.P.; Sumiarsa, D.; Sriwidodo, S. A Review of Fibraurea tinctoria and Its Component, Berberine, as an Antidiabetic and Antioxidant. Molecules 2023, 28, 1294. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Xu, Q.; Ma, J.; Li, X.; Yan, J.; Tian, Y.; Wen, Y.; Chen, T. Berberine and health outcomes: An umbrella review. Phytother. Res. 2023, 37, 2051–2066. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.D.; Zhao, W.; Wang, Z.Z.; Wang, S.K.; Zhou, Z.X.; Song, D.Q.; Wang, Y.M.; et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism 2010, 59, 285–292. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Wang, X.; Liu, S.; Shang, W.; Yuan, G.; Li, F.; Tang, J.; Chen, M.; Chen, J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism 2007, 56, 405–412. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, E.J.; Kim, E.D.; Bayaraa, T.; Frost, S.C.; Hyun, C.K. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes. Biol. Pharm. Bull. 2007, 30, 2120–2125. [Google Scholar] [CrossRef]
- Saha, A.K.; Ruderman, N.B. Malonyl-CoA and AMP-activated protein kinase: An expanding partnership. Mol. Cell. Biochem. 2003, 253, 65–70. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Gong, Z.; Sheng, X.; Li, Z.; Zhang, W.; Qin, Y. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARgamma pathway. Biochem. Biophys. Res. Commun. 2006, 348, 571–578. [Google Scholar] [CrossRef] [PubMed]
- DasNandy, A.; Virge, R.; Hegde, H.V.; Chattopadhyay, D. A review of patent literature on the regulation of glucose metabolism by six phytocompounds in the management of diabetes mellitus and its complications. J. Integr. Med. 2023, 21, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, X.; Shao, L.; Yang, Y.; Shang, W.; Yuan, G.; Jiang, B.; Li, F.; Tang, J.; Jing, H.; et al. Berberine acutely inhibits insulin secretion from beta-cells through 3′,5′-cyclic adenosine 5′-monophosphate signaling pathway. Endocrinology 2008, 149, 4510–4518. [Google Scholar] [CrossRef]
- Kong, W.J.; Zhang, H.; Song, D.Q.; Xue, R.; Zhao, W.; Wei, J.; Wang, Y.M.; Shan, N.; Zhou, Z.X.; Yang, P.; et al. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism 2009, 58, 109–119. [Google Scholar] [CrossRef]
- Galic, S.; Hauser, C.; Kahn, B.B.; Haj, F.G.; Neel, B.G.; Tonks, N.K.; Tiganis, T. Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol. Cell. Biol. 2005, 25, 819–829. [Google Scholar] [CrossRef]
- Goldstein, B.J. Protein-tyrosine phosphatase 1B (PTP1B): A novel therapeutic target for type 2 diabetes mellitus, obesity and related states of insulin resistance. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2001, 1, 265–275. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Huang, C. Berberine inhibits PTP1B activity and mimics insulin action. Biochem. Biophys. Res. Commun. 2010, 397, 543–547. [Google Scholar] [CrossRef]
- Tahtah, Y.; Wubshet, S.G.; Kongstad, K.T.; Heskes, A.M.; Pateraki, I.; Moller, B.L.; Jager, A.K.; Staerk, D. High-resolution PTP1B inhibition profiling combined with high-performance liquid chromatography-high-resolution mass spectrometry-solid-phase extraction-nuclear magnetic resonance spectroscopy: Proof-of-concept and antidiabetic constituents in crude extract of Eremophila lucida. Fitoterapia 2016, 110, 52–58. [Google Scholar] [CrossRef]
- Semaan, D.G.; Igoli, J.O.; Young, L.; Marrero, E.; Gray, A.I.; Rowan, E.G. In vitro anti-diabetic activity of flavonoids and pheophytins from Allophylus cominia Sw. on PTP1B, DPPIV, alpha-glucosidase and alpha-amylase enzymes. J. Ethnopharmacol. 2017, 203, 39–46. [Google Scholar] [CrossRef]
- Yue, S.J.; Liu, J.; Feng, W.W.; Zhang, F.L.; Chen, J.X.; Xin, L.T.; Peng, C.; Guan, H.S.; Wang, C.Y.; Yan, D. System Pharmacology-Based Dissection of the Synergistic Mechanism of Huangqi and Huanglian for Diabetes Mellitus. Front. Pharmacol. 2017, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Farooq, M.A.; Kyunn, W.W. A New Oleanane Type Saponin from the Aerial Parts of Nigella sativa with Anti-Oxidant and Anti-Diabetic Potential. Molecules 2020, 25, 2171. [Google Scholar] [CrossRef]
- Rath, P.; Ranjan, A.; Chauhan, A.; Verma, N.K.; Bhargava, A.; Prasad, R.; Jindal, T. A Critical Review on Role of Available Synthetic Drugs and Phytochemicals in Insulin Resistance Treatment by Targeting PTP1B. Appl. Biochem. Biotechnol. 2022, 194, 4683–4701. [Google Scholar] [CrossRef] [PubMed]
- Akdad, M.; Ameziane, R.; Khallouki, F.; Bakri, Y.; Eddouks, M. Antidiabetic Phytocompounds Acting as Glucose Transport Stimulators. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 147–168. [Google Scholar] [CrossRef]
- Bustanji, Y.; Taha, M.O.; Yousef, A.M.; Al-Bakri, A.G. Berberine potently inhibits protein tyrosine phosphatase 1B: Investigation by docking simulation and experimental validation. J. Enzyme. Inhib. Med. Chem. 2006, 21, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Jin, J.; Liu, P.; Song, Y.; Zhang, H.; Sheng, L.; Zhou, H.; Jiang, B. Berberine Attenuates Hyperglycemia by Inhibiting the Hepatic Glucagon Pathway in Diabetic Mice. Oxid. Med. Cell. Longev. 2020, 2020, 6210526. [Google Scholar] [CrossRef]
- Shu, X.; Li, M.; Cao, Y.; Li, C.; Zhou, W.; Ji, G.; Zhang, L. Berberine Alleviates Non-alcoholic Steatohepatitis Through Modulating Gut Microbiota Mediated Intestinal FXR Activation. Front. Pharmacol. 2021, 12, 750826. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Mechanisms of Disease: Endothelial dysfunction in insulin resistance and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 46–56. [Google Scholar] [CrossRef]
- Mercurio, V.; Pucci, G.; Bosso, G.; Fazio, V.; Battista, F.; Iannuzzi, A.; Brambilla, N.; Vitalini, C.; D’Amato, M.; Giacovelli, G.; et al. A nutraceutical combination reduces left ventricular mass in subjects with metabolic syndrome and left ventricular hypertrophy: A multicenter, randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2020, 39, 1379–1384. [Google Scholar] [CrossRef]
- Meng, S.; Wang, L.S.; Huang, Z.Q.; Zhou, Q.; Sun, Y.G.; Cao, J.T.; Li, Y.G.; Wang, C.Q. Berberine ameliorates inflammation in patients with acute coronary syndrome following percutaneous coronary intervention. Clin. Exp. Pharmacol. Physiol. 2012, 39, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, X.; He, J.; Dai, Z.; Shi, P.; Lu, Y.; Chang, F. The effects of berberine on inflammatory markers in Chinese patients with metabolic syndrome and related disorders: A meta-analysis of randomized controlled trials. Inflammopharmacology 2022, 30, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, X.; Zeng, S.; Tang, W.; Xiao, L.; Zhu, C.; Yu, R. Mechanisms of Berberine for the Treatment of Atherosclerosis Based on Network Pharmacology. Evid. Based Complement. Alternat. Med. 2020, 2020, 3568756. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.H.; Yao, X.Q.; Lau, C.W.; Law, W.I.; Chen, Z.Y.; Kwok, W.; Ho, K.; Huang, Y. Vasorelaxant and antiproliferative effects of berberine. Eur. J. Pharmacol. 2000, 399, 187–196. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Lam, K.S.; Li, Y.; Wong, W.T.; Ye, H.; Lau, C.W.; Vanhoutte, P.M.; Xu, A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc. Res. 2009, 82, 484–492. [Google Scholar] [CrossRef]
- Hong, Y.; Hui, S.C.; Chan, T.Y.; Hou, J.Y. Effect of berberine on regression of pressure-overload induced cardiac hypertrophy in rats. Am. J. Chin. Med. 2002, 30, 589–599. [Google Scholar] [CrossRef]
- Hong, Y.; Hui, S.S.; Chan, B.T.; Hou, J. Effect of berberine on catecholamine levels in rats with experimental cardiac hypertrophy. Life Sci. 2003, 72, 2499–2507. [Google Scholar] [CrossRef]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thevenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jungers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996, 21, 845–853. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Shirwaikar, A.; Rajendran, K.; Punitha, I.S. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull. 2006, 29, 1906–1910. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, X.; Mao, G.; Gao, Z.; Wang, H.; He, Q.; Li, D. Hepatoprotection of berberine against hydrogen peroxide-induced apoptosis by upregulation of Sirtuin 1. Phytother. Res. 2013, 27, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, X.; Yang, R.; Chen, F.; Liao, Y.; Zhu, Z.; Wu, Z.; Sun, X.; Wang, L. Effects of Berberine on the Gastrointestinal Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 588517. [Google Scholar] [CrossRef]
- McVeigh, G.E.; Cohn, J.N. Endothelial dysfunction and the metabolic syndrome. Curr. Diab. Rep. 2003, 3, 87–92. [Google Scholar] [CrossRef]
- Tziomalos, K.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Endothelial dysfunction in metabolic syndrome: Prevalence, pathogenesis and management. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 140–146. [Google Scholar] [CrossRef]
- Dagher, O.; Mury, P.; Thorin-Trescases, N.; Noly, P.E.; Thorin, E.; Carrier, M. Therapeutic Potential of Quercetin to Alleviate Endothelial Dysfunction in Age-Related Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 658400. [Google Scholar] [CrossRef]
- Marzoog, B.A. Recent advances in molecular biology of metabolic syndrome pathophysiology: Endothelial dysfunction as a potential therapeutic target. J. Diabetes Metab. Disord. 2022, 21, 1903–1911. [Google Scholar] [CrossRef]
- Ahirwar, A.K.; Jain, A.; Singh, A.; Goswami, B.; Bhatnagar, M.K.; Bhatacharjee, J. The study of markers of endothelial dysfunction in metabolic syndrome. Horm. Mol. Biol. Clin. Investig. 2015, 24, 131–136. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Monistrol-Mula, A.; Cardellach, F.; Garrabou, G. Nutrition, Bioenergetics, and Metabolic Syndrome. Nutrients 2020, 12, 2785. [Google Scholar] [CrossRef]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Milajerdi, A.; Dadgostar, E.; Kolahdooz, F.; Chamani, M.; Amirani, E.; Mirzaei, H.; Asemi, Z. The Effects of Quercetin Supplementation on Blood Pressures and Endothelial Function among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2019, 25, 1372–1384. [Google Scholar] [CrossRef]
- Tabrizi, R.; Tamtaji, O.R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Dadgostar, E.; Asemi, Z. The effects of quercetin supplementation on lipid profiles and inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1855–1868. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Effect of quercetin supplementation on plasma lipid profiles, blood pressure, and glucose levels: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 615–626. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of quercetin supplementation on glycemic control among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Haddad, P.S. The Antidiabetic Potential of Quercetin: Underlying Mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.K.; Wang, Z.; Ribnicky, D.; Soileau, J.L.; Cefalu, W.T.; Gettys, T.W. Failure of dietary quercetin to alter the temporal progression of insulin resistance among tissues of C57BL/6J mice during the development of diet-induced obesity. Diabetologia 2009, 52, 514–523. [Google Scholar] [CrossRef]
- Chuang, C.C.; Martinez, K.; Xie, G.; Kennedy, A.; Bumrungpert, A.; Overman, A.; Jia, W.; McIntosh, M.K. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-{alpha}-mediated inflammation and insulin resistance in primary human adipocytes. Am. J. Clin. Nutr. 2010, 92, 1511–1521. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Zhou, N.; Wang, X.; Liu, Q.; Bai, Y.; Bai, Y.; Liu, Z.; Yang, H.; Zou, J.; et al. Quercetin improves insulin resistance and hepatic lipid accumulation in vitro in a NAFLD cell model. Biomed. Rep. 2013, 1, 71–76. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Sandeep Varma, R.; Patki, P.S. Quercetin ameliorate insulin resistance and up-regulates cellular antioxidants during oleic acid induced hepatic steatosis in HepG2 cells. Toxicol. Vitr. Vitr. 2013, 27, 945–953. [Google Scholar] [CrossRef]
- Dai, X.; Ding, Y.; Zhang, Z.; Cai, X.; Bao, L.; Li, Y. Quercetin but not quercitrin ameliorates tumor necrosis factor-alpha-induced insulin resistance in C2C12 skeletal muscle cells. Biol. Pharm. Bull. 2013, 36, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Henagan, T.M.; Lenard, N.R.; Gettys, T.W.; Stewart, L.K. Dietary quercetin supplementation in mice increases skeletal muscle PGC1alpha expression, improves mitochondrial function and attenuates insulin resistance in a time-specific manner. PLoS ONE 2014, 9, e89365. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Martinez-Castano, M.G.; Portillo, M.P. Quercetin can reduce insulin resistance without decreasing adipose tissue and skeletal muscle fat accumulation. Genes Nutr. 2014, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boque, N.; Macarulla, M.T.; Portillo, M.P.; Martinez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Porras, D.; Nistal, E.; Martinez-Florez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, D.; Zhang, D.; Bai, L.; Yao, R.; Yu, J.; Cheng, W.; Yu, C. Quercetin Decreases Insulin Resistance in a Polycystic Ovary Syndrome Rat Model by Improving Inflammatory Microenvironment. Reprod. Sci. 2017, 24, 682–690. [Google Scholar] [CrossRef]
- Khodarahmi, A.; Eshaghian, A.; Safari, F.; Moradi, A. Quercetin Mitigates Hepatic Insulin Resistance in Rats with Bile Duct Ligation Through Modulation of the STAT3/SOCS3/IRS1 Signaling Pathway. J. Food Sci. 2019, 84, 3045–3053. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef]
- Tan, Y.; Tam, C.C.; Rolston, M.; Alves, P.; Chen, L.; Meng, S.; Hong, H.; Chang, S.K.C.; Yokoyama, W. Quercetin Ameliorates Insulin Resistance and Restores Gut Microbiome in Mice on High-Fat Diets. Antioxidants 2021, 10, 1251. [Google Scholar] [CrossRef]
- Gorbenko, N.I.; Borikov, O.Y.; Kiprych, T.V.; Ivanova, O.V.; Taran, K.V.; Litvinova, T.S. Quercetin improves myocardial redox status in rats with type 2 diabetes. Endocr. Regul. 2021, 55, 142–152. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, A.; Guru, B.; Ahmad, S.; Gulzar, F.; Kumar, P.; Ahmad, I.; Tamrakar, A.K. Fructose-mediated NLRP3 activation induces inflammation and lipogenesis in adipose tissue. J. Nutr. Biochem. 2022, 107, 109080. [Google Scholar] [CrossRef] [PubMed]
- Er, F.; Zorba, E.; Gunay, M.; Koz, M.; Yilmaz, C.; Pasaoglu, O.T.; Turkozkan, N. Effect of Exercise and Quercetin in Rats with Metabolic Syndrome Induced with Fructose. Metab. Syndr. Relat. Disord. 2022, 20, 57–66. [Google Scholar]
- Jiang, J.; Zhang, G.; Yu, M.; Gu, J.; Zheng, Y.; Sun, J.; Ding, S. Quercetin improves the adipose inflammatory response and insulin signaling to reduce “real-world” particulate matter-induced insulin resistance. Environ. Sci. Pollut. Res. Int. 2022, 29, 2146–2157. [Google Scholar] [CrossRef]
- Jiao, Y.; Williams, A.; Wei, N. Quercetin ameliorated insulin resistance via regulating METTL3-mediated N6-methyladenosine modification of PRKD2 mRNA in skeletal muscle and C2C12 myocyte cell line. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2655–2668. [Google Scholar] [CrossRef]
- Su, L.; Zeng, Y.; Li, G.; Chen, J.; Chen, X. Quercetin improves high-fat diet-induced obesity by modulating gut microbiota and metabolites in C57BL/6J mice. Phytother. Res. 2022, 12, 4558–4572. [Google Scholar] [CrossRef]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxid. Med. Cell. Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef]
- Yan, L.; Vaghari-Tabari, M.; Malakoti, F.; Moein, S.; Qujeq, D.; Yousefi, B.; Asemi, Z. Quercetin: An effective polyphenol in alleviating diabetes and diabetic complications. Crit. Rev. Food Sci. Nutr. 2022, 1–24. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.H.; Neufer, P.D. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol. Metab. 2012, 23, 142–153. [Google Scholar] [CrossRef]
- Guo, X.D.; Zhang, D.Y.; Gao, X.J.; Parry, J.; Liu, K.; Liu, B.L.; Wang, M. Quercetin and quercetin-3-O-glucuronide are equally effective in ameliorating endothelial insulin resistance through inhibition of reactive oxygen species-associated inflammation. Mol. Nutr. Food Res. 2013, 57, 1037–1045. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Cano-Martinez, A.; Diaz-Diaz, E.; Manzano-Pech, L.; Gamas-Magana, A.; Castrejon-Tellez, V.; Tapia-Cortina, C.; Perez-Torres, I. Resveratrol and Quercetin Administration Improves Antioxidant DEFENSES and reduces Fatty Liver in Metabolic Syndrome Rats. Molecules 2019, 24, 1297. [Google Scholar] [CrossRef]
- Kabirifar, R.; Ghoreshi, Z.A.; Safari, F.; Karimollah, A.; Moradi, A.; Eskandari-Nasab, E. Quercetin protects liver injury induced by bile duct ligation via attenuation of Rac1 and NADPH oxidase1 expression in rats. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 88–95. [Google Scholar] [CrossRef]
- Liu, X.; Song, L. Quercetin protects human liver cells from o,p’-DDT-induced toxicity by suppressing Nrf2 and NADPH oxidase-regulated ROS production. Food Chem. Toxicol. 2022, 161, 112849. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jiang, C.; Mei, G.; Zhao, Y.; Chen, L.; Liu, J.; Tang, Y.; Gao, C.; Yao, P. Quercetin Alleviates Ferroptosis of Pancreatic beta Cells in Type 2 Diabetes. Nutrients 2020, 12, 2954. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P. The superoxide-forming enzymatic system of phagocytes. Free Radic. Biol. Med. 1988, 4, 225–261. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Hartlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Teuber, J.P.; Essandoh, K.; Hummel, S.L.; Madamanchi, N.R.; Brody, M.J. NADPH Oxidases in Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction. Antioxidants 2022, 11, 1822. [Google Scholar] [CrossRef]
- Nabeebaccus, A.A.; Reumiller, C.M.; Shen, J.; Zoccarato, A.; Santos, C.X.C.; Shah, A.M. The regulation of cardiac intermediary metabolism by NADPH oxidases. Cardiovasc. Res. 2023, 118, 3305–3319. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.G.; Cheon, J.; Yoon, D.H.; Park, H.S.; Kim, H.K.; Kim, J.J.; Koh, S.K. Allium sativum potentiates suicide gene therapy for murine transitional cell carcinoma. Nutr. Cancer 2000, 38, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [CrossRef]
- Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Couture, P.; Perusse, L.; Vohl, M.C. SREBF1 gene variations modulate insulin sensitivity in response to a fish oil supplementation. Lipids Health Dis. 2014, 13, 152. [Google Scholar] [CrossRef]
- Seo, Y.S.; Kang, O.H.; Kim, S.B.; Mun, S.H.; Kang, D.H.; Yang, D.W.; Choi, J.G.; Lee, Y.M.; Kang, D.K.; Lee, H.S.; et al. Quercetin prevents adipogenesis by regulation of transcriptional factors and lipases in OP9 cells. Int. J. Mol. Med. 2015, 35, 1779–1785. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhang, Z.C.; Hassan, W.; Li, Y.; Liu, J.; Shang, J. Amelioration of free fatty acid-induced fatty liver by quercetin-3-O-beta-D-glucuronide through modulation of peroxisome proliferator-activated receptor-alpha/sterol regulatory element-binding protein-1c signaling. Hepatol. Res. 2016, 46, 225–238. [Google Scholar] [CrossRef]
- Jayachandran, M.; Zhang, T.; Wu, Z.; Liu, Y.; Xu, B. Isoquercetin regulates SREBP-1C via AMPK pathway in skeletal muscle to exert antihyperlipidemic and anti-inflammatory effects in STZ induced diabetic rats. Mol. Biol. Rep. 2020, 47, 593–602. [Google Scholar] [CrossRef]
- Saleh Al-Maamari, J.N.; Rahmadi, M.; Panggono, S.M.; Prameswari, D.A.; Pratiwi, E.D.; Ardianto, C.; Balan, S.S.; Suprapti, B. The effects of quercetin on the expression of SREBP-1c mRNA in high-fat diet-induced NAFLD in mice. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 637–644. [Google Scholar] [CrossRef]
- Xie, M.; Gao, L.; Liu, Z.; Yuan, R.; Zhuoma, D.; Tsering, D.; Wang, Y.; Huang, S.; Li, B. Malus toringoides (Rehd.) Hughes Ameliorates Nonalcoholic Fatty Liver Disease with Diabetes via Downregulation of SREBP-1c and the NF-kappaB Pathway In Vivo and In Vitro. J. Med. Food 2022, 25, 1112–1125. [Google Scholar] [CrossRef]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef]
- Goldstein, B.J.; Bittner-Kowalczyk, A.; White, M.F.; Harbeck, M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J. Biol. Chem. 2000, 275, 4283–4289. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef]
- Shaik, Y.B.; Castellani, M.L.; Perrella, A.; Conti, F.; Salini, V.; Tete, S.; Madhappan, B.; Vecchiet, J.; De Lutiis, M.A.; Caraffa, A.; et al. Role of quercetin (a natural herbal compound) in allergy and inflammation. J. Biol. Regul. Homeost. Agents 2006, 20, 47–52. [Google Scholar] [PubMed]
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Chen, G.W.; Chen, Y.C.; Shen, C.K.; Lu, D.Y.; Yang, L.Y.; Chen, J.H.; Yeh, W.L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients 2021, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandris, C.; Lauro, R.; Presta, I.; Sesti, G. C-reactive protein induces phosphorylation of insulin receptor substrate-1 on Ser307 and Ser 612 in L6 myocytes, thereby impairing the insulin signalling pathway that promotes glucose transport. Diabetologia 2007, 50, 840–849. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [CrossRef]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Therapeutic potential of quercetin in an animal model of PCOS: Possible involvement of AMPK/SIRT-1 axis. Eur. J. Pharmacol. 2021, 900, 174062. [Google Scholar] [CrossRef]
- Khorshidi, M.; Moini, A.; Alipoor, E.; Rezvan, N.; Gorgani-Firuzjaee, S.; Yaseri, M.; Hosseinzadeh-Attar, M.J. The effects of quercetin supplementation on metabolic and hormonal parameters as well as plasma concentration and gene expression of resistin in overweight or obese women with polycystic ovary syndrome. Phytother. Res. 2018, 32, 2282–2289. [Google Scholar] [CrossRef]
- Rezvan, N.; Moini, A.; Gorgani-Firuzjaee, S.; Hosseinzadeh-Attar, M.J. Oral Quercetin Supplementation Enhances Adiponectin Receptor Transcript Expression in Polycystic Ovary Syndrome Patients: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Cell J. 2018, 19, 627–633. [Google Scholar] [CrossRef]
- Rezvan, N.; Moini, A.; Janani, L.; Mohammad, K.; Saedisomeolia, A.; Nourbakhsh, M.; Gorgani-Firuzjaee, S.; Mazaherioun, M.; Hosseinzadeh-Attar, M.J. Effects of Quercetin on Adiponectin-Mediated Insulin Sensitivity in Polycystic Ovary Syndrome: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Horm. Metab. Res. 2017, 49, 115–121. [Google Scholar] [CrossRef]
- Chen, T.; Jia, F.; Yu, Y.; Zhang, W.; Wang, C.; Zhu, S.; Zhang, N.; Liu, X. Potential Role of Quercetin in Polycystic Ovary Syndrome and Its Complications: A Review. Molecules 2022, 27, 4476. [Google Scholar] [CrossRef]
- Ma, C.; Xiang, Q.; Song, G.; Wang, X. Quercetin and polycystic ovary syndrome. Front. Pharmacol. 2022, 13, 1006678. [Google Scholar] [CrossRef]
- Das, S.; Roy, P.; Pal, R.; Auddy, R.G.; Chakraborti, A.S.; Mukherjee, A. Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS ONE 2014, 9, e101818. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef]
- MacDonald-Ramos, K.; Michan, L.; Martinez-Ibarra, A.; Cerbon, M. Silymarin is an ally against insulin resistance: A review. Ann. Hepatol. 2021, 23, 100255. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- van der Wijst, M.G.; Brown, R.; Rots, M.G. Nrf2, the master redox switch: The Achilles’ heel of ovarian cancer? Biochim. Biophys. Acta 2014, 1846, 494–509. [Google Scholar] [CrossRef]
- Rolo, A.P.; Oliveira, P.J.; Moreno, A.J.; Palmeira, C.M. Protection against post-ischemic mitochondrial injury in rat liver by silymarin or TUDC. Hepatol. Res. 2003, 26, 217–224. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, L.J.; Tashiro, S.; Onodera, S.; Uchiumi, F.; Ikejima, T. Silibinin protects rat cardiac myocyte from isoproterenol-induced DNA damage independent on regulation of cell cycle. Biol. Pharm. Bull. 2006, 29, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Azadpour, M.; Farajollahi, M.M.; Dariushnejad, H.; Varzi, A.M.; Varezardi, A.; Barati, M. Effects of synthetic silymarin-PLGA nanoparticles on M2 polarization and inflammatory cytokines in LPS-treated murine peritoneal macrophages. Iran. J. Basic Med. Sci. 2021, 24, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, M.Y.; Jeon, Y.J. Silymarin Inhibits Morphological Changes in LPS-Stimulated Macrophages by Blocking NF-kappaB Pathway. Korean J. Physiol. Pharmacol. 2015, 19, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, E.S.; Wagoner, J.; MacDonald, J.; Bammler, T.; Bruckner, J.; Brownell, J.; Beyer, R.P.; Zink, E.M.; Kim, Y.M.; Kyle, J.E.; et al. Silymarin Suppresses Cellular Inflammation By Inducing Reparative Stress Signaling. J. Nat. Prod. 2015, 78, 1990–2000. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Jeon, Y.J.; Kim, H.M.; Han, S.H.; Yang, K.H. Inhibition of inducible nitric-oxide synthase expression by silymarin in lipopolysaccharide-stimulated macrophages. J. Pharmacol. Exp. Ther. 2002, 302, 138–144. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Wang, Y.; Zhu, T. Silymarin improved diet-induced liver damage and insulin resistance by decreasing inflammation in mice. Pharm. Biol. 2016, 54, 2995–3000. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Feng, B.; Huang, B.; Jing, Y.; Shen, S.; Feng, W.; Wang, W.; Meng, R.; Zhu, D. Silymarin ameliorates the disordered glucose metabolism of mice with diet-induced obesity by activating the hepatic SIRT1 pathway. Cell. Signal. 2021, 84, 110023. [Google Scholar] [CrossRef]
- Qiao, L.; Shao, J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J. Biol. Chem. 2006, 281, 39915–39924. [Google Scholar] [CrossRef]
- Hollenberg, A.N.; Susulic, V.S.; Madura, J.P.; Zhang, B.; Moller, D.E.; Tontonoz, P.; Sarraf, P.; Spiegelman, B.M.; Lowell, B.B. Functional antagonism between CCAAT/Enhancer binding protein-alpha and peroxisome proliferator-activated receptor-gamma on the leptin promoter. J. Biol. Chem. 1997, 272, 5283–5290. [Google Scholar] [CrossRef]
- Iwaki, M.; Matsuda, M.; Maeda, N.; Funahashi, T.; Matsuzawa, Y.; Makishima, M.; Shimomura, I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 2003, 52, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.; Lorenz, K.; Braithwaite, S.S.; Colca, J.R.; Palazuk, B.J.; Hotamisligil, G.S.; Spiegelman, B.M. Altered gene expression for tumor necrosis factor-alpha and its receptors during drug and dietary modulation of insulin resistance. Endocrinology 1994, 134, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Tomaru, T.; Steger, D.J.; Lefterova, M.I.; Schupp, M.; Lazar, M.A. Adipocyte-specific expression of murine resistin is mediated by synergism between peroxisome proliferator-activated receptor gamma and CCAAT/enhancer-binding proteins. J. Biol. Chem. 2009, 284, 6116–6125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Yang, Y.R.; Mo, Z.J.; Ding, Y.; Jiang, W.J. Silibinin improves palmitate-induced insulin resistance in C2C12 myotubes by attenuating IRS-1/PI3K/Akt pathway inhibition. Braz. J. Med. Biol. Res. 2015, 48, 440–446. [Google Scholar] [CrossRef]
- Bouderba, S.; Sanchez-Martin, C.; Villanueva, G.R.; Detaille, D.; Koceir, E.A. Beneficial effects of silibinin against the progression of metabolic syndrome, increased oxidative stress, and liver steatosis in Psammomys obesus, a relevant animal model of human obesity and diabetes. J. Diabetes 2014, 6, 184–192. [Google Scholar] [CrossRef]
- Soto, C.; Mena, R.; Luna, J.; Cerbon, M.; Larrieta, E.; Vital, P.; Uria, E.; Sanchez, M.; Recoba, R.; Barron, H.; et al. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004, 75, 2167–2180. [Google Scholar] [CrossRef]
- Soto, C.; Raya, L.; Perez, J.; Gonzalez, I.; Perez, S. Silymarin induces expression of pancreatic Nkx6.1 transcription factor and beta-cells neogenesis in a pancreatectomy model. Molecules 2014, 19, 4654–4668. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, J.; Lee, M.Y.; Sudhanva, M.S.; Devakumar, S.; Jeon, Y.J. Silymarin Inhibits Cytokine-Stimulated Pancreatic Beta Cells by Blocking the ERK1/2 Pathway. Biomol. Ther. 2014, 22, 282–287. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef]
- Cao, R.; Cronk, Z.X.; Zha, W.; Sun, L.; Wang, X.; Fang, Y.; Studer, E.; Zhou, H.; Pandak, W.M.; Dent, P.; et al. Bile acids regulate hepatic gluconeogenic genes and farnesoid X receptor via G(alpha)i-protein-coupled receptors and the AKT pathway. J. Lipid Res. 2010, 51, 2234–2244. [Google Scholar] [CrossRef]
- Kabir, M.; Catalano, K.J.; Ananthnarayan, S.; Kim, S.P.; Van Citters, G.W.; Dea, M.K.; Bergman, R.N. Molecular evidence supporting the portal theory: A causative link between visceral adiposity and hepatic insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E454–E461. [Google Scholar] [CrossRef]
- Yao, J.; Zhi, M.; Gao, X.; Hu, P.; Li, C.; Yang, X. Effect and the probable mechanisms of silibinin in regulating insulin resistance in the liver of rats with non-alcoholic fatty liver. Braz. J. Med. Biol. Res. 2013, 46, 270–277. [Google Scholar] [CrossRef]
- Elgarf, A.T.; Mahdy, M.M.; Ali Sabri, N. Effect of Silymarin Supplementation on Glycemic Control, Lipid Profile and Insulin Resistance in Patients with Type 2 Diabetes Mellitus. Int. J. Adv. Res. 2015, 3, 812–821. [Google Scholar]
- Ebrahimpour-Koujan, S.; Gargari, B.P.; Mobasseri, M.; Valizadeh, H.; Asghari-Jafarabadi, M. Lower glycemic indices and lipid profile among type 2 diabetes mellitus patients who received novel dose of Silybum marianum (L.) Gaertn. (silymarin) extract supplement: A Triple-blinded randomized controlled clinical trial. Phytomedicine 2018, 44, 39–44. [Google Scholar] [CrossRef]
- Memon, A.; Siddiqui, S.S.; Ata, M.A.; Shaikh, K.R.; Soomro, U.A.; Shaikh, S. Silymarin improves glycemic control through reduction of insulin resistance in newly diagnosed patients of type 2 diabetes mellitus. Prof. Med. J. 2022, 29, 362–366. [Google Scholar]
- Ravari, S.S.; Talaei, B.; Gharib, Z. The effects of silymarin on type 2 diabetes mellitus: A systematic review and meta-analysis. Obes. Med. 2021, 26, 100368. [Google Scholar] [CrossRef]
- Xiao, F.; Gao, F.; Zhou, S.; Wang, L. The therapeutic effects of silymarin for patients with glucose/lipid metabolic dysfunction: A meta-analysis. Medicine 2020, 99, e22249. [Google Scholar] [CrossRef]
- Li Volti, G.; Salomone, S.; Sorrenti, V.; Mangiameli, A.; Urso, V.; Siarkos, I.; Galvano, F.; Salamone, F. Effect of silibinin on endothelial dysfunction and ADMA levels in obese diabetic mice. Cardiovasc. Diabetol. 2011, 10, 62. [Google Scholar] [CrossRef]
- Demirci, B.; Demir, O.; Dost, T.; Birincioglu, M. Treated effect of silymarin on vascular function of aged rats: Dependant on nitric oxide pathway. Pharm. Biol. 2013, 52, 453–457. [Google Scholar] [CrossRef]
- Sugamura, K.; Keaney, J.F., Jr. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef]
- Taleb, A.; Ahmad, K.A.; Ihsan, A.U.; Qu, J.; Lin, N.; Hezam, K.; Koju, N.; Hui, L.; Qilong, D. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed. Pharmacother. 2018, 102, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.R.; Viswanath, R.K. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp. Clin. Cardiol. 2007, 12, 179–187. [Google Scholar] [PubMed]
- Jessup, W.; Dean, R.T.; de Whalley, C.V.; Rankin, S.M.; Leake, D.S. The role of oxidative modification and antioxidants in LDL metabolism and atherosclerosis. Adv. Exp. Med. Biol. 1990, 264, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.M.; Briggs, J.P. Silymarin for diabetic nephropathy: The challenges of botanical product research. Am. J. Kidney Dis. 2012, 60, 887–889. [Google Scholar] [CrossRef]
- Soto, C.; Perez, J.; Garcia, V.; Uria, E.; Vadillo, M.; Raya, L. Effect of silymarin on kidneys of rats suffering from alloxan-induced diabetes mellitus. Phytomedicine 2010, 17, 1090–1094. [Google Scholar] [CrossRef]
- Garcia-Ramirez, M.; Turch, M.; Simo-Servat, O.; Hernandez, C.; Simo, R. Silymarin prevents diabetes-induced hyperpermeability in human retinal endothelial cells. Endocrinol. Diabetes Nutr. 2018, 65, 200–205. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Juarez-Fernandez, M.; Porras, D.; Petrov, P.; Roman-Saguillo, S.; Garcia-Mediavilla, M.V.; Soluyanova, P.; Martinez-Florez, S.; Gonzalez-Gallego, J.; Nistal, E.; Jover, R.; et al. The Synbiotic Combination of Akkermansia muciniphila and Quercetin Ameliorates Early Obesity and NAFLD through Gut Microbiota Reshaping and Bile Acid Metabolism Modulation. Antioxidants 2021, 10, 2001. [Google Scholar] [CrossRef]
- Williamson, G.; Sheedy, K. Effects of Polyphenols on Insulin Resistance. Nutrients 2020, 12, 3135. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. J. Pharm. Anal. 2022, 12, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Y.; Liu, Y.; Hou, L.; Li, S.; Tian, H.; Zhao, T. Berberine Modulates Gut Microbiota and Reduces Insulin Resistance via the TLR4 Signaling Pathway. Exp. Clin. Endocrinol. Diabetes 2018, 126, 513–520. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martinez-Florez, S.; Olcoz, J.L.; Jover, R.; Jorquera, F.; Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S. Functional Interactions between Gut Microbiota Transplantation, Quercetin, and High-Fat Diet Determine Non-Alcoholic Fatty Liver Disease Development in Germ-Free Mice. Mol. Nutr. Food Res. 2019, 63, e1800930. [Google Scholar] [CrossRef]
- Liu, W.; Zhi, A. The potential of Quercetin to protect against loperamide-induced constipation in rats. Food Sci. Nutr. 2021, 9, 3297–3307. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Xing, Y.; Xing, R.; Liu, Y.; Xu, Y. Changes of gut microbiota during silybin-mediated treatment of high-fat diet-induced non-alcoholic fatty liver disease in mice. Hepatol. Res. 2020, 50, 5–14. [Google Scholar] [CrossRef]
- Sun, W.L.; Hua, S.; Li, X.Y.; Shen, L.; Wu, H.; Ji, H.F. Microbially produced vitamin B12 contributes to the lipid-lowering effect of silymarin. Nat. Commun. 2023, 14, 477. [Google Scholar] [CrossRef]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What causes the insulin resistance underlying obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87. [Google Scholar] [CrossRef]
- Sebekova, K.; Gurecka, R.; Csongova, M.; Koborova, I.; Repiska, G.; Podracka, L. Lean insulin-resistant young adults display increased cardiometabolic risk: A retrospective cross-sectional study. Diabetes Res. Clin. Pract. 2022, 185, 109217. [Google Scholar] [CrossRef]
- Gast, K.B.; Tjeerdema, N.; Stijnen, T.; Smit, J.W.; Dekkers, O.M. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: Meta-analysis. PLoS ONE 2012, 7, e52036. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.P.; Lamarche, B.; Mauriege, P.; Cantin, B.; Dagenais, G.R.; Moorjani, S.; Lupien, P.J. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 1996, 334, 952–957. [Google Scholar] [CrossRef]
- Pyorala, M.; Miettinen, H.; Laakso, M.; Pyorala, K. Hyperinsulinemia and the risk of stroke in healthy middle-aged men: The 22-year follow-up results of the Helsinki Policemen Study. Stroke 1998, 29, 1860–1866. [Google Scholar] [CrossRef]
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef]
- Pereira, T.M.; Pimenta, F.S.; Porto, M.L.; Baldo, M.P.; Campagnaro, B.P.; Gava, A.L.; Meyrelles, S.S.; Vasquez, E.C. Coadjuvants in the Diabetic Complications: Nutraceuticals and Drugs with Pleiotropic Effects. Int. J. Mol. Sci. 2016, 17, 1273. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P. The role of selected nutraceuticals in management of prediabetes and diabetes: An updated review of the literature. Phytother. Res. 2022, 36, 3709–3765. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed. Pharmacother. 2018, 104, 465–473. [Google Scholar] [CrossRef]
- McAuley, K.A.; Williams, S.M.; Mann, J.I.; Goulding, A.; Chisholm, A.; Wilson, N.; Story, G.; McLay, R.T.; Harper, M.J.; Jones, I.E. Intensive lifestyle changes are necessary to improve insulin sensitivity: A randomized controlled trial. Diabetes Care 2002, 25, 445–452. [Google Scholar] [CrossRef] [PubMed]



| Compound | Position of Key-Word Compound | Insulin Resistance in Title | Insulin Resistance in Abstract |
|---|---|---|---|
| Berberine | Title | 53 | 192 |
| Abstract | 62 | 268 | |
| Quercetin | Title | 19 | 100 |
| Abstract | 38 | 244 | |
| Silymarin | Title | 6 | 31 |
| Abstract | 15 | 68 | |
| Polyphenols | Title | 28 | 139 |
| Abstract | 98 | 591 | |
| Resveratrol | Title | 60 | 281 |
| Abstract | 82 | 467 | |
| Catechin | Title | 1 | 9 |
| Abstract | 38 | 189 |
| Model | Dose of Qtn | Main Effects | Ref. |
|---|---|---|---|
| IR induced by high-fat diet (mouse) | 1.5% (wt/wt) in diet for 8 weeks | No improvement | [100] |
| IR induced by TNFα in primary human adipocytes | 10–30 µmol/L in vitro | ↓ NF-kB and cytokine secretion ↓ PTP1B gene expression | [101] |
| NAFLD model and IR induced by fatty acids in hepatic HepG2 cell line culture | 0.1–100 µmol/L in vitro | ↑ phosphorylation of insulin-signaling pathway (IRβ e IRS-1) ↓ sterol regulatory element-binding protein-1c (SREBP-1c) and fatty acid synthase (FAS) | [102] |
| Steatosis-like phenotype and IR induced by oleic acid in hepatic HepG2 cell line culture | 1–10 µmol/L in vitro | ↑ insulin mediated glucose uptake ↑ glutathione content ↓TNF-α, IL-8 and lipid peroxides | [103] |
| IR in skeletal muscle cells treated with TNFα | 10–20 µmol/L in vitro | ↑ glucose absorption ↑ MAPK e Akt (PKB) phosphorylation ↓ NF-kB and INOS | [104] |
| IR induced by high-fat diet (mouse) | 50 µg/die/mouse (low dose) or 600 µg/die/mouse (high dose) for 8 weeks | ↓ IR (Low dose only). ↑ Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) | [105] |
| IR induced by high-fat and high-sugar diet (rat) | 30 mg/kg for 6 weeks | ↓ fruttosamine, basal glycemia, insulin e HOMA-IR No differences in free fatty acid concentrations and obesity indices. | [106] |
| IR and obesity induced by high-fat and high-sugar diet (rat) | Added to diet 30 mg/kg/die | Prevents IR ↓ body weight Attenuates intestinal dysbiosis | [107] |
| High-fat diet induced metabolic syndrome (mouse) | Added to diet 0.05% (wt/wt) for 16 weeks | ↓ IR ↓ lipoperoxidation ↓ LPS-mediated inflammation Restores microbiota (Firmicutes/Bacteroidetes equilibrium) | [108] |
| IR in dehydroepiandrosterone-induced polycystic ovary syndrome (rat) | 100 mg/kg for 28 days | Recovery of the estrous cycle ↓ insulinemia ↓ TLR4, NF-kB and IL-1beta ↓ expression of p22phox (NADPH oxidase) | [109] |
| IR and liver fibrosis induced by bile duct ligation (BDL) (rat) | 30 mg/kg/day for 4 weeks after operation | Antidiabetic effect: ↓ STAT3 e SOCS3, with ↑ IRS-1. Antifibrotic effect: ↓ Rac1-GTP, Rac1, HIF-1alpha, NOX1 and others | [110] |
| Myotubes L6 in vitro | 1–10 µmol/L | ↑ increased translocation of GLUT4 and glucose uptake ↑IRS-1/PI3K/Akt reporting | [111] |
| Normal ICR mice | 10–100 mg/kg (quercetin-3-O-β-glucoside) | ↑ increase of GLUT4 in skeletal muscle | [111] |
| C57BL/6J mice fed high-fat diet | 0.05% in the diet for 6 weeks | ↓ hyperglycemia, obesity and steatosis ↓ insulin and leptin ↑ Akkermansia and Bacteroidetes/Firmicutes ratio in feces ↓ expression of Srebf1, Ppara, Cyp51, Scd1 and Fasn genes | [112] |
| T2DM induced with high-calorie diet and streptozotocin (rat) | 10–50 mg/kg for 8 weeks | ↑ insulin sensitivity ↓ oxidative stress in cardiac mitochondria ↓ NADPH oxidase and xanthine oxidase ↑ superoxide dismutase, glutathione peroxidase, glutathione reductase | [113] |
| Myotubes from healthy donors | 10 μMol/L (in vitro) | ↑ MAPK, IRS-1, and AS160 phosphorylation in basal conditions and ↑ glycogen synthase kinase 3 (GSK3beta) in insulin-stimulated conditions | [33] |
| IR and inflammation induced by 60% fructose diet (rat) | 100 mg/kg for 6 weeks | ↑ glucose tolerance ↓ adipose tissue ↓ NLRP3 inflammasome ↓ IL-1β and IL-18 | [114] |
| Metabolic syndrome and IR induced by 20% fructose (rat) | 15 mg/kg/die | ↓ glycemia and insulinemia ↓ systolic arterial pressure, triglycerides, cholesterol VLDL | [115] |
| IR induced by chronic exposure to PM2.5, with elevation of serum IL-6 and TNF-α and activation of NLRP3 (mouse). | 50–100 mg/kg for 18 weeks | ↓ glycemia and IR ↓ systemic inflammation ↓ NLRP3 in adipocytes | [116] |
| IR induced by high-fat diet (mouse) | 10 mg/kg (gavage) for 10 weeks | ↓ glycemia and IR ↓ ROS production ↑ SOD e GSH | [117] |
| IR induced in C2C12 myocytes by palmitic acid (PA) | 5–10 µmol/L | ↓ methyladenosine (m6A), METTL3 and p-IRS-1 ↑ PRKD2, GLUT4 e p-Akt expression ↓ oxidative stress | [117] |
| IR and obesity induced by HFD (mouse) | 50 mg/kg for 20 weeks | ↓ Inflammation of adipose tissue ↑ glucose tolerance Changes in gut microbiota | [118] |
| IR induced in HepG2 hepatic cells by PA | 4–8 µmol/L | ↑ GLUT4 and glucose uptake ↑ glycogen production ↓ Ser612 phosphorylation of IRS-1 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellavite, P.; Fazio, S.; Affuso, F. A Descriptive Review of the Action Mechanisms of Berberine, Quercetin and Silymarin on Insulin Resistance/Hyperinsulinemia and Cardiovascular Prevention. Molecules 2023, 28, 4491. https://doi.org/10.3390/molecules28114491
Bellavite P, Fazio S, Affuso F. A Descriptive Review of the Action Mechanisms of Berberine, Quercetin and Silymarin on Insulin Resistance/Hyperinsulinemia and Cardiovascular Prevention. Molecules. 2023; 28(11):4491. https://doi.org/10.3390/molecules28114491
Chicago/Turabian StyleBellavite, Paolo, Serafino Fazio, and Flora Affuso. 2023. "A Descriptive Review of the Action Mechanisms of Berberine, Quercetin and Silymarin on Insulin Resistance/Hyperinsulinemia and Cardiovascular Prevention" Molecules 28, no. 11: 4491. https://doi.org/10.3390/molecules28114491