LC-MS Profiling of Kakkonto and Identification of Ephedrine as a Key Component for Its Anti-Glycation Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Anti-Glycation Activity of 147 Oral Kampo Prescriptions
2.2. Identification of Chemical Constituents in Kakkonto by LC-MS
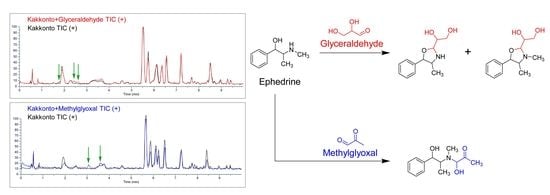
2.3. Identification of the Components That Contribute to the Anti-Glycation Activity
2.4. Evaluation of Ephedrae Herba Extract for Anti-Glycation Activity
3. Materials and Methods
3.1. General Methods
3.2. Materials and Chemicals
3.3. Sample Preparation of Kampo Solutions
3.4. Assay for the Anti-Glycation Activity Using D-Ribose
3.5. Assay for the Anti-Glycation Activity Using Glyceraldehyde
3.6. UHPLC-MS Conditions
3.7. Preparation of Kakkonto Solution for LC-MS
3.8. Evaluation of the GA and MGO Trapping Capacity of Kakkonto
3.9. Extraction and Assay for the Anti-Glycation Activity of Ephedrae Herba
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Welsh, K.J.; Kirkman, M.S.; Sacks, D.B. Role of glycated proteins in the diagnosis and management of diabetes: Research gaps and future directions. Diabetes Care 2016, 39, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Lapolla, A.; Fedele, D.; Reitano, R.; Bonfante, L.; Guizzo, M.; Seraglia, R.; Tubaro, M.; Traldi, P. Mass spectrometric study of in vivo production of advanced glycation endproducts/peptides. J. Mass Spectrom. 2005, 40, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Lapolla, A.; Fedele, D.; Seraglia, R.; Traldi, P. The role of mass spectrometry in the study of non-enzymatic protein glycation in diabetes: An update. Mass Spectrom. Rev. 2006, 25, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A Perspective on the maillard reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef]
- Sjoblom, N.M.; Kelsey, M.M.G.; Scheck, R.A. A Systematic study of selective protein glycation. Angew. Chem. Int. Ed. 2018, 57, 16077–16082. [Google Scholar] [CrossRef]
- Nagaraj, R.H.; Shipanova, I.N.; Faust, F.M. Protein crosslinking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J. Biol. Chem. 1996, 271, 19338–19345. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Baynes, J.W. The role of AGEs in aging: Causation or correlation. Exp. Gerontol. 2001, 36, 1527–1537. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kikuchi, S.; Sasaki, N.; Suzuki, T.; Watai, T.; Iwaki, M.; Bucala, R.; Yamagishi, S. Involvement of advanced glycation end-products (AGEs) in Alzheimer’s disease. Curr. Alzheimer Res. 2004, 1, 39–46. [Google Scholar] [CrossRef]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Beisswenger, P.; Ruggiero-Lopez, D. Metformin inhibition of glycation processes. Diabetes Metab. 2003, 29, 6S95–6S103. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 1999, 48, 198–202. [Google Scholar] [CrossRef]
- Freedman, B.I.; Wuerth, J.-P.; Cartwright, K.; Bain, R.P.; Dippe, S.; Hershon, K.; Mooradian, A.D.; Spinowitz, B.S. Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control. Clin. Trials 1999, 20, 493–510. [Google Scholar] [CrossRef]
- Tsuge, A.; Watanabe, A.; Kodama, Y.; Hisaka, S.; Nose, M. Orengedokuto exerts anti-allergic effects via inhibition of effector T cell activation in a murine model of contact hypersensitivity. J. Nat. Med. 2022, 76, 144–151. [Google Scholar] [CrossRef]
- Toume, K.; Hou, Z.; Yu, H.; Kato, M.; Maesaka, M.; Bai, Y.; Hanazawa, S.; Ge, Y.; Andoh, T.; Komatsu, K. Search of anti-allodynic compounds from Plantaginis Semen, a crude drug ingredient of Kampo formula “Goshajinkigan”. J. Nat. Med. 2019, 73, 761–768. [Google Scholar] [CrossRef]
- Takiyama, M.; Matsumoto, T.; Sanechika, S.; Watanabe, J. Pharmacokinetic study of traditional Japanese Kampo medicine shimotsuto used to treat gynecological diseases in rats. J. Nat. Med. 2021, 75, 361–371. [Google Scholar] [CrossRef]
- Onoda, T.; Li, W.; Higai, K.; Koike, K. Evaluation of 147 Kampo prescriptions as novel protein tyrosine phosphatase 1B (PTP1B) inhibitory agents. BMC Complement. Altern. Med. 2014, 14, 64. [Google Scholar] [CrossRef]
- Sato, N.; Li, W.; Takemoto, H.; Takeuchi, M.; Nakamura, A.; Tokura, E.; Akahane, C.; Ueno, K.; Komatsu, K.; Kuriyama, N.; et al. Comprehensive evaluation of antioxidant effects of Japanese Kampo medicines led to identification of Tsudosan formulation as a potent antioxidant agent. J. Nat. Med. 2019, 73, 163–172. [Google Scholar] [CrossRef]
- Onoda, T.; Li, W.; Sasaki, T.; Miyake, M.; Higai, K.; Koike, K. Identification and evaluation of magnolol and chrysophanol as the principle protein tyrosine phosphatase-1B inhibitory compounds in a Kampo medicine, Masiningan. J. Ethnopharmacol. 2016, 186, 84–90. [Google Scholar] [CrossRef]
- Onoda, T.; Ishikawa, C.; Fukazawa, T.; Li, W.; Obayashi, M.; Koike, K. Inhibitory activities of selected Kampo formulations on human aldose reductase. BMC Complement. Altern. Med. 2014, 14, 435/1–435/6. [Google Scholar] [CrossRef] [PubMed]
- Derbre, S.; Gatto, J.; Pelleray, A.; Coulon, L.; Seraphin, D.; Richomme, P. Automating a 96-well microtiter plate assay for identification of AGEs inhibitors or inducers: Application to the screening of a small natural compounds library. Anal. Bioanal. Chem. 2010, 398, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Kikuchi, A.; Yamaya, M.; Deng, X.; Sugawara, M.; Takayama, S.; Nagatomi, R.; Ishii, T. Kakkonto inhibits cytokine production induced by rhinovirus infection in primary cultures of human nasal epithelial cells. Front. Pharmacol. 2021, 12, 687818. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.-J.; Wang, Q.; Bo, T.; Ye, M.; Qiao, X.; Yang, W.-Z.; Xiang, C.; Guan, X.-Y.; Guo, D.-A. Rapid characterization of chemical constituents and rats metabolites of the traditional Chinese patent medicine Gegen-Qinlian-Wan by UHPLC/DAD/qTOF-MS. J. Pharm. Biomed. Anal. 2013, 72, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Jiang, Y.; Li, W.; Cai, B. Rapid characterization and determination of isoflavones and triterpenoid saponins in Fu-Zhu-Jiang-Tang tablets using UHPLC-Q-TOF/MS and HPLC-UV. Anal. Methods 2016, 8, 4211–4219. [Google Scholar] [CrossRef]
- Shi, Y.-H.; Zhu, S.; Ge, Y.-W.; Toume, K.; Wang, Z.; Batkhuu, J.; Komatsu, K. Characterization and quantification of monoterpenoids in different types of peony root and the related Paeonia species by liquid chromatography coupled with ion trap and time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2016, 129, 581–592. [Google Scholar] [CrossRef]
- Huang, W.-W.; Wang, M.-Y.; Shi, H.-M.; Peng, Y.; Peng, C.-S.; Zhang, M.; Li, Y.; Lu, J.; Li, X.-B. Comparative study of bioactive constituents in crude and processed Glycyrrhizae radix and their respective metabolic profiles in gastrointestinal tract in vitro by HPLC-DAD and HPLC-ESI/MS analyses. Arch. Pharmacal Res. 2012, 35, 1945–1952. [Google Scholar] [CrossRef]
- Cheng, M.; Ding, L.; Kan, H.; Zhang, H.; Jiang, B.; Sun, Y.; Cao, S.; Li, W.; Koike, K.; Qiu, F. Isolation, structural elucidation and in vitro hepatoprotective activity of flavonoids from Glycyrrhiza uralensis. J. Nat. Med. 2019, 73, 847–854. [Google Scholar] [CrossRef]
- Kim, J.-H.; Shin, H.-K.; Seo, C.-S. Chemical interaction between Paeonia lactiflora and Glycyrrhiza uralensis, the components of Jakyakgamcho-tang, using a validated high-performance liquid chromatography method: Herbal combination and chemical interaction in a decoction. J. Sep. Sci. 2014, 37, 2704–2715. [Google Scholar] [CrossRef]
- Xu, T.; Yang, M.; Li, Y.; Chen, X.; Wang, Q.; Deng, W.; Pang, X.; Yu, K.; Jiang, B.; Guan, S.; et al. An integrated exact mass spectrometric strategy for comprehensive and rapid characterization of phenolic compounds in licorice. Rapid Commun. Mass Spectrom. 2013, 27, 2297–2309. [Google Scholar] [CrossRef]
- Song, W.; Qiao, X.; Chen, K.; Wang, Y.; Ji, S.; Feng, J.; Li, K.; Lin, Y.; Ye, M. Biosynthesis-Based Quantitative Analysis of 151 Secondary Metabolites of Licorice To Differentiate Medicinal Glycyrrhiza Species and Their Hybrids. Anal. Chem. 2017, 89, 3146–3153. [Google Scholar] [CrossRef]
- Zhang, Z.-T.; Guo, N.; Zhuang, G.-D.; Deng, S.-M.; He, W.-J.; Chen, Z.-Q.; Xu, Y.-H.; Tang, D.; Wang, S.-M. Metabolic Profiling of Carbonyl Compounds for Unveiling Protective Mechanisms of Pueraria lobata against Diabetic Nephropathy by UPLC-Q-Orbitrap HRMS/MS Analysis. J. Agric. Food Chem. 2021, 69, 10943–10951. [Google Scholar] [CrossRef]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive Constituents of Glycyrrhiza uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef]
- Lee, S.J.; Baek, H.J.; Lee, C.H.; Kim, H.P. Antiinflammatory activity of isoflavonoids from Pueraria radix and biochanin A derivatives. Arch. Pharmacal Res. 1994, 17, 31–35. [Google Scholar] [CrossRef]
- Maciejewska-Turska, M.; Pecio, L.; Zgorka, G. Isolation of Mirificin and Other Bioactive Isoflavone Glycosides from the Kudzu Root Lyophilisate Using Centrifugal Partition and Flash Chromatographic Techniques. Molecules 2022, 27, 6227. [Google Scholar] [CrossRef]
- Sun, Y.-G.; Wang, S.-S.; Feng, J.-T.; Xue, X.-Y.; Liang, X.-M. Two new isoflavone glycosides from Pueraria lobata. J. Asian Nat. Prod. Res. 2008, 10, 719–723. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Jo, M.S.; Lee, D.; Baek, S.-E.; Baek, J.; Yu, J.S.; Jo, J.; Yun, H.; Kang, K.S.; Yoo, J.-E.; et al. Dual effects of isoflavonoids from Pueraria lobata roots on estrogenic activity and anti-proliferation of MCF-7 human breast carcinoma cells. Bioorg. Chem. 2019, 83, 135–144. [Google Scholar] [CrossRef]
- Nohara, T.; Kinjo, J.; Furusawa, J.; Sakai, Y.; Inoue, M.; Shirataki, Y.; Ishibashi, Y.; Yokoe, I.; Komatsu, M. But-2-enolides from Pueraria lobata and revised structures of puerosides A, B and sophoroside A. Phytochemistry 1993, 33, 1207–1210. [Google Scholar] [CrossRef]
- Shi, Y.-H.; Zhu, S.; Ge, Y.-W.; He, Y.-M.; Kazuma, K.; Wang, Z.; Yoshimatsu, K.; Komatsu, K. Monoterpene derivatives with anti-allergic activity from red peony root, the root of Paeonia lactiflora. Fitoterapia 2016, 108, 55–61. [Google Scholar] [CrossRef]
- Zheng, Y.-F.; Wei, J.-H.; Fang, S.-Q.; Tang, Y.-P.; Cheng, H.-B.; Wang, T.-L.; Li, C.-Y.; Peng, G.-P. Hepatoprotective triterpene saponins from the roots of Glycyrrhiza inflata. Molecules 2015, 20, 6273–6283. [Google Scholar] [CrossRef]
- Beckett, A.H.; Jones, G.R. Identification and stereochemistry of (2S,4S,5R)- and (2R,4S,5R)-2,3,4-trimethyl-5-phenyloxazolidine, degradation products of ephedrine. Tetrahedron 1977, 33, 3313–3316. [Google Scholar] [CrossRef]
- Xiu, L.-M.; Miura, A.B.; Yamamoto, K.; Kobayashi, T.; Song, Q.-H.; Kitamura, H.; Cyong, J.-C. Pancreatic islet regeneration by ephedrine in mice with streptozotocin-induced diabetes. Am. J. Chin. Med. 2001, 29, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Hajleh, M.N.A.; Khleifat, K.M.; Alqaraleh, M.; Al-Hraishat, E.A.; Al-limoun, M.O.; Qaralleh, H.; Al-Dujaili, E.A.S. Antioxidant and Antihyperglycemic Effects of Ephedra foeminea Aqueous Extract in Streptozotocin-Induced Diabetic Rats. Nutrients 2022, 14, 2338. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-K.; Um, J.-Y.; Jang, H.-J.; Lee, B.-C. Beneficial effect of dietary Ephedra sinica on obesity and glucose intolerance in high-fat diet-fed mice. Exp. Ther. Med. 2012, 3, 707–712. [Google Scholar] [CrossRef]













| Glycation Inhibitory Ratio (%) ± SE | |||
|---|---|---|---|
| No. | Kampo Prescriptions | BSA–D-Ribose Assay | BSA–GA Assay |
| 50 μU/mL | 400 μU/mL | ||
| 1 | Shiniseihaito | 111.9 ± 6.2 | 68.2 ± 0.87 |
| 2 | Kakkonto | 87.5 ± 5.2 | 111.0 ± 4.0 |
| 3 | Daisaikotokyodaio | 81.8 ± 1.9 | 60.6 ± 0.46 |
| 4 | Saikokeishikankyoto | 74.0 ± 5.2 | 54.6 ± 0.64 |
| 5 | Keishikashakuyakuto | 73.0 ± 5.2 | 55.7 ± 0.9 |
| 6 | Gorinsan | 72.7 ± 6.0 | 56.2 ± 0.98 |
| 7 | Sammotsuogonto | 71.4 ± 4.4 | 65.6 ± 1.4 |
| 8 | Unseiin | 71.2 ± 7.0 | 82.3 ± 1.9 |
| 9 | Ninjinyoeito | 64.6 ± 12.8 | <50 |
| 10 | Saibokuto | 62.0 ± 7.3 | <50 |
| 11 | Shosaikoto | 62.0 ± 4.2 | 75.3 ± 12.5 |
| 12 | Tsudosan | 57.4 ± 14.8 | 89.0 ± 1.1 |
| 13 | Sansoninto | 56.9 ± 5.7 | <50 |
| 14 | Otsujito | 56.6 ± 5.6 | 65.0 ± 1.4 |
| 15 | Saikanto | 55.8 ± 2.9 | 53.0 ± 6.8 |
| 16 | Saikokeishito | 55.7 ± 9.8 | 60.6 ± 3.3 |
| 17 | Hangeshashinto | 52.3 ± 2.4 | 60.4 ± 1.3 |
| 18 | Bakumondoto | 50.1 ± 7.8 | <50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, K.; Kikuchi, T.; Ikube, K.; Otsuki, K.; Koike, K.; Li, W. LC-MS Profiling of Kakkonto and Identification of Ephedrine as a Key Component for Its Anti-Glycation Activity. Molecules 2023, 28, 4409. https://doi.org/10.3390/molecules28114409
Ito K, Kikuchi T, Ikube K, Otsuki K, Koike K, Li W. LC-MS Profiling of Kakkonto and Identification of Ephedrine as a Key Component for Its Anti-Glycation Activity. Molecules. 2023; 28(11):4409. https://doi.org/10.3390/molecules28114409
Chicago/Turabian StyleIto, Kaori, Takashi Kikuchi, Kanako Ikube, Kouharu Otsuki, Kazuo Koike, and Wei Li. 2023. "LC-MS Profiling of Kakkonto and Identification of Ephedrine as a Key Component for Its Anti-Glycation Activity" Molecules 28, no. 11: 4409. https://doi.org/10.3390/molecules28114409