Polypropylene Nanocomposites Attained by In Situ Polymerization Using SBA-15 Particles as Support for Metallocene Catalysts: Effect of Molecular Weight and Tacticity on Crystalline Details, Phase Transitions and Rheological Behavior
Abstract
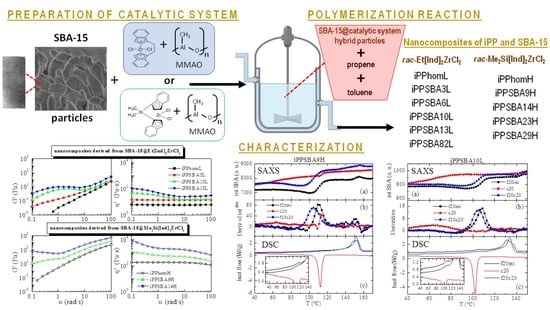
:1. Introduction
2. Results and Discussion
2.1. Polymerizations and Microstructure of the Obtained Nanocomposites
2.2. Thermal Stability and Morphological Characteristics
2.3. Phase Transitions and Crystalline Details
2.4. Rheological Response
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of the Supported Catalyst
Impregnation of Pre-Activated Zr Catalysts Using MAO on SBA-15 (PA Method)
3.3. Propylene Polymerizations
3.4. Characterization of Homopolymers and Nanocomposites
3.4.1. Size Exclusion Chromatography
3.4.2. Nuclear Magnetic Resonance
3.4.3. Preparation of Films
3.4.4. Thermogravimetric Analysis
3.4.5. Scanning Electron Microscopy
3.4.6. Differential Scanning Calorimetry Analysis
3.4.7. X-ray Experiments with Conventional and Synchrotron Radiation
3.4.8. Rheological Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, X.; Zheng, G.; Dai, K.; Jia, Z.; Li, S.; Liu, C.H.; Chen, J.; Shen, C.H.; Li, Q. Morphological comparison of isotactic polypropylene molded by water-assisted and conventional injection molding. J. Mater. Sci. 2011, 46, 7830–7838. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Shi, S.; Liu, C.; Dai, K.; Yin, R.; Schubert, D.W.; Zheng, G.; Shen, C. Annealing Induced Mechanical Reinforcement of Injection Molded iPP Parts. Macromol. Mater. Eng. 2016, 301, 1468–1472. [Google Scholar] [CrossRef]
- Pan, Y.; Guo, X.; Zheng, G.; Liu, C.; Chen, Q.; Shen, C.; Liu, X. Shear-Induced Skin-Core Structure of Molten Isotactic Polypropylene and the Formation of β-Crystal. Macromol. Mater. Eng. 2018, 303, 1800083. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Lian, M.; Pan, Y.; Chen, Q.; Liu, H.; Zheng, G.; Guo, Z.; Schubert, D.W.; Shen, C.; et al. Self-reinforcing and toughening isotactic polypropylene via melt sequential injection molding. Polym. Test. 2018, 67, 183–189. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Qin, Y.; Voigt, M.; Liu, X.; Zheng, G.; Chen, Q.; Schubert, D.W.; Liu, C.; Shen, C. Creep and recovery behavior of injection–molded isotactic polypropylene with controllable skin–core structure. Polym. Test. 2018, 69, 478–484. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Benavente, R.; Pérez, E. Influence of thermal history on morphology and viscoelastic behavior of ethylene-1-octene copolymers synthesized with metallocene catalysts. J. Mater. Res. 2001, 16, 1103–1111. [Google Scholar] [CrossRef]
- Severn, J.R.; Chadwick, J.C. Tailor-Made Polymers: Via Immobilization of Alpha-Olefin Polymerization Catalysts; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Prieto, O.; Pereña, J.M.; Benavente, R.; Pérez, E.; Cerrada, M.L. Viscoelastic relaxation mechanisms of conventional polypropylene toughened by a plastomer. J. Polym. Sci. Polym. Phys. 2003, 41, 1878–1888. [Google Scholar] [CrossRef]
- Vlasblom, M. Handbook of Properties of Textile and Technical Fibres; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Röttger, M.; Domenech, T.; van der Weegen, R.; Breuillac, A.; Nicolae, R.; Leibler, L. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 2017, 356, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Eagan, J.M.; Xu, J.; Girolamo, R.D.; Thurber, C.M.; Macosko, C.W.; LaPointe, A.M.; Bates, F.S.; Coates, G.W. Combining polyethylene and polypropylene: Enhanced performance with PE/iPP multiblock polymers. Science 2017, 355, 814–816. [Google Scholar] [CrossRef]
- Naga, N.; Shiono, T.; Ikeda, T. Copolymerization of Propene and Nonconjugated Diene Involving Intramolecular Cyclization with Metallocene/Methylaluminoxane. Macromolecules 1999, 32, 1348–1355. [Google Scholar] [CrossRef]
- Kaminsky, W.; Laban, A. Metallocene catalysis. Appl. Catalysis A General 2001, 222, 47–61. [Google Scholar] [CrossRef]
- Kukral, J.; Lehmus, P.; Feifel, T.; Troll, C.; Rieger, B. Dual-side ansa-zirconocene dichlorides for high molecular weight isotactic polypropene elastomers. Organometallics 2000, 19, 3767–3775. [Google Scholar] [CrossRef]
- Brintzinger, H.H.; Fischer, D.; Mulhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific Olefin Polymerization with Chiral Metallocene Catalysts. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- Palza, H.; López-Majada, J.M.; Quijada, R.; Perena, J.M.; Benavente, R.; Pérez, E.; Cerrada, M.L. Comonomer Length Influence on the Structure and Mechanical Response of Metallocenic Polypropylenic Materials. Macromol. Chem. Phys. 2008, 209, 2259–2267. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Corradini, P.; Tarallo, O.; Dello Iacono, S.; Ciaccia, E.; Resconi, L. Crystal Structure of the Trigonal Form of Isotactic Polypropylene as an Example of Density-Driven Polymer Structure. J. Am. Chem. Soc. 2006, 128, 80–81. [Google Scholar] [CrossRef]
- Polo-Corpa, M.J.; Benavente, R.; Velilla, T.; Quijada, R.; Pérez, E.; Cerrada, M.L. Development of the mesomorphic phase in isotactic propene/higher a-olefin copolymers at intermediate comonomer content and its effect on properties. Eur. Polym. J. 2010, 46, 1345–1354. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Talarico, G.; de Ballesteros, O.R. Structure of isotactic propylene-pentene copolymers. Macromolecules 2007, 40, 8531–8532. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Elvira, J.M.; Pérez, E.; Cerrada, M.L. Isotactic Poly(propylene-co-1-pentene-co-1-hexene) Terpolymers: Synthesis, Molecular Characterization and Evidence of the Trigonal Polymorph. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3251–3259. [Google Scholar] [CrossRef]
- Arranz-Andrés, J.; Parrilla, R.; Cerrada, M.L.; Pérez, E. Mesophase Formation in Random Propylene-co-1-Octene Copolymers. Macromolecules 2013, 46, 8557–8568. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez Elvira, J.M.; Lorenzo, V.; Pérez, E.; Cerrada, M.L. Synthesis, molecular characterization, evaluation of polymorphic behavior and indentation response in isotactic poly(propylene-co-1-heptene) copolymers. Eur. Polym. J. 2015, 64, 52–61. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Elvira, J.M.; Barranco-García, R.; Pérez, E.; Cerrada, M.L. Trigonal form as a tool for tuning mechanical behavior in poly(propylene-co-1-pentene-co-1-heptene) terpolymers. Polymer 2016, 99, 112–121. [Google Scholar] [CrossRef]
- Werny, M.J.; Zarupski, J.; ten Have, I.C.; Piovano, A.; Hendriksen, C.; Friederichs, N.H.; Meirer, F.; Groppo, E.; Weckhuysen, B.M. Correlating the Morphological Evolution of Individual Catalyst Particles to the Kinetic Behavior of Metallocene-Based Ethylene Polymerization Catalysts. JACS Au 2021, 1, 1996–2008. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Kim, H.J.; Shiono, T. Polymerization of propene with highly isospecific. SiO2-supported zirconocene catalysts activated with common alkylaluminiums. Macromol. Chem. Phys. 1994, 195, 3347–3360. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Zucchi, D.; Tritto, I.; Locatelli, P.; Dall’Occo, T. Silica-supported metallocenes: Stereochemical comparison between homogeneous and heterogeneous catalysis. Macromol. Rapid Commun. 1995, 16, 581–590. [Google Scholar] [CrossRef]
- Semikolenova, N.; Zakharov, V.A. On the interaction of supported zirconocene catalysts with alkylaluminium co-catalysts. Macromol. Chem. Phys. 1997, 198, 2889–2897. [Google Scholar] [CrossRef]
- Moreira, S.C.; Marques, M.F.V. Polyethylene synthesis using zeolite as support for metallocene catalyst. Eur. Polym. J. 2001, 37, 2123–2130. [Google Scholar] [CrossRef]
- Huang, Y.; Qin, Y.; Zhou, Y.; Niu, H.; Yu, Z.-Z.; Dong, J.-Y. Polypropylene/Graphene Oxide Nanocomposites Prepared by In Situ Ziegler−Natta Polymerization. Chem. Mater. 2010, 22, 4096–4102. [Google Scholar] [CrossRef]
- Wang, N.; Qin, Y.; Huang, Y.; Niu, H.; Dong, J.-Y.; Wang, Y. Functionalized multi-walled carbon nanotubes with stereospecific Ziegler-Natta catalyst species: Towards facile in situ preparation of polypropylene nanocomposites. Appl. Catal. A General 2012, 435–436, 107–114. [Google Scholar] [CrossRef]
- Milani, M.A.; Quijada, R.; Basso, N.R.S.; Graebin, A.P.; Galland, G.B. Influence of the graphite type on the synthesis of polypropylene/graphene nanocomposites. Polym. Sci. Part A Polym. Chem. 2012, 50, 3598–3605. [Google Scholar] [CrossRef]
- Milani, M.A.; González, D.; Quijada, R.; Basso, N.R.S.; Cerrada, M.L.; Azambuja, D.S.; Galland, G.B. Polypropylene/graphene nanosheet nanocomposites by in situ polymerization: Synthesis, characterization and fundamental properties. Compos. Sci. Technol. 2013, 84, 1–7. [Google Scholar] [CrossRef]
- Cecílio, D.M.; Cerrada, M.L.; Pérez, E.; Fernandes, A.; Lourenço, J.P.; McKenna, T.F.; Ribeiro, M.R. Unique stiffness-deformability features of dendrimeric silica reinforced HDPE nanocomposites obtained by an innovative route. Microporous Mesoporous Mater. 2022, 331, 111619. [Google Scholar] [CrossRef]
- Cecílio, D.M.; Cerrada, M.L.; Pérez, E.; Fernandes, A.; Lourenço, J.P.; McKenna, T.F.; Ribeiro, M.R. A novel approach for preparation of nanocomposites with an excellent rigidity/deformability balance, based on reinforced HDPE with halloysite. Eur. Polym. J. 2023, 184, 111765. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.-W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Pérez, E.; Lourenço, J.P.; Campos, J.M.; Ribeiro, M.R. HDPE/MCM-41 Nanocomposites: Crystalline Structure and Viscoelastic Behaviour. Microporous Mesoporous Mater. 2010, 130, 215–223. [Google Scholar] [CrossRef]
- Kurek, A.; Mark, S.; Enders, M.; Kristen, M.O.; Mülhaupt, R. Mesoporous Silica Supported Multiple Single-Site Catalysts and Polyethylene Reactor Blends with Tailor-Made Trimodal and Ultra-Broad Molecular Weight Distributions. Macromol. Rapid Commun. 2010, 31, 1359–1363. [Google Scholar] [CrossRef]
- Campos, J.M.; Lourenço, J.P.; Cramail, H.; Ribeiro, M.R. Nanostructured silica materials in olefin polymerisation: From catalytic behaviour to polymer characteristics. Prog. Polym. Sci. 2012, 37, 1764–1804. [Google Scholar] [CrossRef]
- Dong, X.C.; Wang, L.; Jiang, G.H.; Zhao, Z.R.; Sun, T.X.; Yu, H.J.; Wang, W.Q. MCM-41 and SBA-15 supported Cp2ZrCl2 catalysts for the preparation of nano-polyethylene fibres via in situ ethylene extrusion polymerization. J. Mol. Catal. A Chem. 2005, 240, 239–244. [Google Scholar]
- Campos, J.M.; Ribeiro, M.R.; Lourenço, J.P.; Fernandes, A. Ethylene polymerisation with zirconocene supported in Al-modified MCM-41: Catalytic behaviour and polymer properties. J. Mol. Catal. A−Chem. 2007, 277, 93–101. [Google Scholar] [CrossRef]
- Campos, J.M.; Paulo Lourenço, J.; Pérez, E.; Cerrada, M.L.; Ribeiro, M.R. Self-reinforced Hybrid Polyethylene/MCM-41 Nanocomposites: In-situ Polymerisation and Effect of MCM-41 Content on Rigidity. J. Nanosci. Nanotech. 2009, 9, 3966–3974. [Google Scholar] [CrossRef]
- Bento, A.; Lourenço, J.P.; Fernandes, A.; Ribeiro, M.R.; Arranz-Andrés, J.; Lorenzo, V.; Cerrada, M.L. Gas permeability properties of decorated MCM 41/polyethylene hybrids prepared by in situ polymerization. J. Membr. Sci. 2012, 415–416, 702–711. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Pérez, E.; Lourenço, J.P.; Bento, A.; Ribeiro, M.R. Decorated MCM-41/polyethylene hybrids: Crystalline Details and Viscoelastic Behavior. Polymer 2013, 54, 2611–2620. [Google Scholar] [CrossRef]
- Bento, A.; Lourenço, J.P.; Fernandes, A.; Cerrada, M.L.; Ribeiro, M.R. Functionalization of mesoporous MCM-41 (nano)particles: Preparation methodologies, role on catalytic features and dispersion within PE nanocomposites. Chem. Cat. Chem. 2013, 5, 966–976. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Bento, A.; Pérez, E.; Lorenzo, V.; Lourenço, J.P.; Ribeiro, M.R. Hybrid materials based on polyethylene and MCM-41 microparticles functionalized with silanes: Catalytic aspects of in situ polymerization. crystalline features and mechanical properties. Microporous Mesoporous Mater. 2016, 232, 86–96. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Cerrada, M.L.; Pérez, E.; Lorenzo, V.; Cramail, H.; Lourenço, J.P.; Ribeiro, M.R. UHMWPE/SBA-15 nanocomposites synthesized by in situ polymerization. Microporous Mesoporous Mater. 2016, 232, 13–25. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Cerrada, M.L.; Pérez, E.; Lorenzo, V.; Vallés, E.; Ressia, J.; Cramail, H.; Lourenço, J.P.; Ribeiro, M.R. UHMWPE/HDPE in-reactor blends, prepared by in situ polymerization: Synthetic aspects and characterization. Express Polym. Lett. 2017, 11, 344–361. [Google Scholar] [CrossRef]
- Marques, M.F.V.; Pombo, C.C.; Silva, R.A.; Conte, A. Binary metallocene supported catalyst for propylene polymerization. Eur. Polym. J. 2003, 39, 561–567. [Google Scholar] [CrossRef]
- Vayá, V.I.C.; Belelli, P.G.; dos Santos, J.H.Z.; Ferreira, M.L.; Damiani, D.E. Influence of acidic support in metallocene catalysts for ethylene polymerization. J. Catal. 2001, 204, 1–10. [Google Scholar] [CrossRef]
- Li, K.T.; Ko, F.S. Dimethylsilylbis(1-indenyl) zirconium dichloride/methylaluminoxane catalyst supported on nanosized silica for propylene polymerization. J. Appl. Polym. Sci. 2008, 107, 1387–1394. [Google Scholar] [CrossRef]
- González, D.M.; Quijada, R.; Yazdani-Pedram, M.; Lourenço, J.P.; Ribeiro, M.R. Preparation of polypropylene-based nanocomposites using nanosized MCM-41 as support and in situ polymerization. Polym. Int. 2016, 65, 320–326. [Google Scholar] [CrossRef]
- Hoyos, M.; Tiemblo, P.; Gómez-Elvira, J.M. The role of microstructure. molar mass and morphology on local relaxations in isotactic polypropylene. The α relaxation. Polymer 2007, 48, 183–194. [Google Scholar] [CrossRef]
- Díez-Rodríguez, T.M.; Blázquez-Blázquez, E.; Fernández-García, M.; Muñoz-Bonilla, A.; Pérez, E.; Cerrada, M.L. Biocidal mesoporous SBA-15 particles decorated with Ag nanowires: Nucleant role in PLLA crystallization and antimicrobial transfer of their activity to the resultant biobased PLLA-SBA15@Ag composites. Microporous Mesoporous Mater. 2023, 352, 112493. [Google Scholar] [CrossRef]
- Barranco-García, R.; Ferreira, A.E.; Ribeiro, M.R.; Lorenzo, V.; García-Peñas, A.; Gómez-Elvira, J.M.; Pérez, E.; Cerrada, M.L. Hybrid materials obtained by in situ polymerization based on polypropylene and mesoporous SBA-15 silica particles: Catalytic aspects, crystalline details and mechanical behavior. Polymer 2018, 151, 218–230. [Google Scholar] [CrossRef]
- Kaminsky, W.; Renner, F. High melting polypropylenes by silica-supported zirconocene catalysts. Makromol. Chem. Rapid Commun. 1993, 14, 239–243. [Google Scholar] [CrossRef]
- Franceschini, F.C.; Tavares, T.T.D.; Greco, P.P.; Bianchini, D.; Stedile, F.C.; Galland, G.B.; dos Santos, J.H.Z.; Soares, J.B.P. Polypropylene obtained with in situ supported metallocene catalysts. J. Mol. Catal. A-Chem. 2003, 202, 127–134. [Google Scholar] [CrossRef]
- Chien, J.C.W. Supported Metallocene Polymerization Catalysis in Metallocen-Based Polyolefins; Scheirs, J., Kaminsky, W., Eds.; Chapter 8; Wiley Series in Polymer Science: Hoboken, NJ, USA, 1999; Volume 1, pp. 173–199. [Google Scholar]
- Barranco-García, R.; Cerrada, M.L.; Ressia, J.A.; Vallés, E.M.; García-Peñas, A.; Pérez, E.; Gómez-Elvira, J.M. Effect of mesoporous SBA-15 silica on the thermal stability of isotactic polypropylene based nanocomposites prepared by melt extrusion. Polym. Degrad. Stab. 2018, 154, 211–221. [Google Scholar] [CrossRef]
- Martínez, M.C.; Benavente, R.; Gómez-Elvira, J.M. Molecular weight dependence and stereoselective chain cleavage during the early stages of the isotactic polypropylene pyrolysis. Polym. Degrad. Stab. 2017, 143, 26–34. [Google Scholar] [CrossRef]
- Achimsky, L.; Audouin, L.; Verdu, J.; Rychlá, L.; Rychlý, J. The effect of oxygen pressure on the rate of polypropylene oxidation determined by chemiluminescence. Eur. Polym. J. 1999, 35, 557–563. [Google Scholar] [CrossRef]
- Deryło-Marczewska, A.; Zienkiewicz-Strzałka, M.; Skrzypczyńska, K.; Światkowski, A.; Kuśmierek, K. Evaluation of the SBA-15 materials ability to accumulation of 4-chlorophenol on carbon paste electrode. Adsorption 2016, 22, 801–812. [Google Scholar] [CrossRef]
- Barranco-García, R.; Gómez-Elvira, J.M.; Ressia, J.A.; Quinzani, L.; Vallés, E.M.; Pérez, E.; Cerrada, M.L. Effect of iPP molecular weight on its confinement within mesoporous SBA-15 silica in extruded iPP-SBA-15 nanocomposites. Microporous Mesoporous Mater. 2020, 294, 109945. [Google Scholar] [CrossRef]
- Barranco-García, R.; Gómez-Elvira, J.M.; Ressia, J.A.; Quinzani, L.; Vallés, E.M.; Pérez, E.; Cerrada, M.L. Variation of Ultimate Properties in Extruded iPP-Mesoporous Silica Nanocomposites by Effect of iPP Confinement within the Mesostructures. Polymers 2020, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Díez-Rodríguez, T.M.; Blázquez-Blázquez, E.; Antunes, N.L.C.; Ribeiro, M.R.; Pérez, E.; Cerrada, M.L. Confinement in Extruded Nanocomposites Based on PCL and Mesoporous Silicas: Effect of Pore Sizes and Their Influence in Ultimate Mechanical Response. J. Compos. Sci. 2021, 5, 321. [Google Scholar] [CrossRef]
- Natta, G.; Corradini, P. Strucutre and properties of isotactic polypropylene. Nuovo Cim. Suppl. 1960, 15, 40–51. [Google Scholar] [CrossRef]
- Turner-Jones, A.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Die Makromol. Chem. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Brückner, S.; Meille, S.V.; Petraccone, V.; Pirozzi, B. Polymorphism in isotactic polypropylene. Prog. Polym. Sci. 1991, 16, 361–404. [Google Scholar] [CrossRef]
- Varga, J.J. β-Modification of isotactic polypropylene: Preparation. structure.processing. properties. and application. Macromol. Sci. Phys. B 2002, 41, 1121–1171. [Google Scholar] [CrossRef]
- Hosier, I.L.; Alamo, R.G.; Esteso, P.; Isasi, J.R.; Mandelkern, L. Formation of the α and γ Polymorphs in Random Metallocene−Propylene Copolymers. Effect of Concentration and Type of Comonomer. Macromolecules 2003, 36, 5623–5636. [Google Scholar] [CrossRef]
- Poon, B.; Rogunova, M.; Hiltner, A.; Baer, E.; Chum, S.P.; Galeski, A.; Piorkowska, E. Structure and properties of homogeneous copolymers of propylene and 1-hexene. Macromolecules 2005, 38, 1232–1243. [Google Scholar] [CrossRef]
- De Rosa, C.; Dello Iacono, S.; Auriemma, F.; Ciaccia, E.; Resconi, L. Crystal structure of isotactic propylene-hexene copolymers: The trigonal form of isotactic polypropylene. Macromolecules 2006, 39, 6098–6109. [Google Scholar] [CrossRef]
- De Rosa, C.; de Ballesteros, O.R.; Auriemma, F.; Di Caprio, M.R. Crystal Structure of the Trigonal Form of Isotactic Propylene-Pentene Copolymers: An Example of the Principle of Entropy-Density Driven Phase Formation in Polymers. Macromolecules 2012, 45, 2749–2763. [Google Scholar] [CrossRef]
- Pérez, E.; Gómez-Elvira, J.M.; Benavente, R.; Cerrada, M.L. Tailoring the formation rate of the mesophase in random propylene-co-1-pentene copolymers. Macromolecules 2012, 45, 6481–6490. [Google Scholar] [CrossRef]
- Boragno, L.; Stagnaro, P.; Forlini, F.; Azzurri, F.; Alfonso, G.C. The trigonal form of i-PP in random C3/C5/C6 terpolymers. Polymer 2013, 54, 1656–1662. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Elvira, J.M.; Lorenzo, V.; Pérez, E.; Cerrada, M. Unprecedented dependence of stiffness parameters and crystallinity on comonomercontent in rapidly cooled propylene-co-1-pentene copolymers. Polymer 2017, 130, 17–25. [Google Scholar] [CrossRef]
- Arranz-Andrés, J.; Peña, B.; Benavente, R.; Pérez, E.; Cerrada, M.L. Influence of Isotacticity and Molecular Weight on the Properties of Metallocenic Isotactic Polypropylene. Eur. Polym. J. 2007, 43, 2357–2370. [Google Scholar] [CrossRef]
- Turner-Jones, A. Development of the γ-Crystal Form in Random Copolymers of Propylene and their Analysis by DSC And X-Ray Methods. Polymer 1971, 12, 487–508. [Google Scholar] [CrossRef]
- Krache, R.; Benavente, R.; López-Majada, J.M.; Pereña, J.M.; Cerrada, M.L.; Pérez, E. Competition between α, β and γ polymorphs in a β-nucleated metallocenic isotactic polypropylene. Macromolecules 2007, 40, 6871–6878. [Google Scholar] [CrossRef]
- Baltá-Calleja, F.J.; Vonk, C.G. X-ray Scattering of Synthetic Polymers; Elservier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Ryan, A.J.; Stanford, J.L.; Bras, W.; Nye, T.M.W. A synchrotron X-ray study of melting and recrystallization in isotactic polypropylene. Polymer 1997, 38, 759–768. [Google Scholar] [CrossRef]
- Crist, B. Small-angle X-ray scattering and polymer melting: A model study. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2454–2460. [Google Scholar] [CrossRef]
- De la Fuente, J.L.; Wilhelm, M.; Spiess, H.W.; Madruga, E.L.; Fernández García, M.; Cerrada, M.L. Thermal. morphological and rheological characterization of poly(acrylic acid-g-styrene) amphiphilic graft copolymers. Polymer 2005, 46, 4544–4553. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered Hydrothermally Stable Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Barranco-García, R.; López-Majada, J.M.; Martínez, J.C.; Gómez-Elvira, J.M.; Pérez, E.; Cerrada, M.L. Confinement of iPP crystallites within mesoporous SBA-15 channels in extruded iPP-SBA-15 nanocomposites by Small Angle X-ray Scattering. Microporous Mesoporous Mater. 2018, 272, 209–216. [Google Scholar] [CrossRef]
- Díez-Rodríguez, T.M.; Blázquez-Blázquez, E.; Antunes, N.L.C.; Ribeiro, M.R.; Pérez, E.; Cerrada, M.L. Nanocomposites of PCL and SBA-15 particles prepared by extrusion: Structural characteristics, confinement of PCL chains within SBA-15 nanometric channels and mechanical behavior. Polymers 2022, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, X.; Song, C.h.; Locke, D.R.; Siefert, S.; Winans, R.E.; Möllmer, J.; Lange, M.; Möller, A.; Gläser, R. Molecular basket sorbents polyethylenimine-SBA-15 for CO2 capture from flue gas: Characterization and sorption properties. Microporous Mesoporous Mater. 2013, 169, 103–111. [Google Scholar] [CrossRef]
- Sauer, J.; Marlow, F.; Schüth, F. Simulation of powder diffraction patterns of modified ordered mesoporous materials. Phys. Chem. Chem. Phys. 2001, 3, 5579–5584. [Google Scholar] [CrossRef]
- Hammond, W.; Prouzet, E.; Mahanti, S.D.; Pinnavaia, T.J. Structure factor for the periodic walls of mesoporous MCM-41 molecular sieves. Microporous Mesoporous Mater. 1999, 27, 19–25. [Google Scholar] [CrossRef]
- Dealy, J.M.; Wang, J. Melt Rheology and Its Applications in the Plastics Industry, 2nd ed.; Springer Science & Business Media: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Gupta, R.K.; Kennel, E.; Kim, K.-J. Polymer Nanocomposites Handbook; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Zhang, Q.; Archer, L.A. Poly(ethylene oxide)/Silica Nanocomposites: Structure and Rheology. Langmuir 2002, 18, 10435–10442. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, Q. Concepts and conflicts in nanoparticles reinforcement to polymers beyond hydrodynamics. Prog. Mater. Sci. 2016, 84, 1–58. [Google Scholar] [CrossRef]
- Bond, E.B.; Spruiell, J.E.; Lin, J.S. A WAXD/SAXS/DSC study on the melting behavior of Ziegler–Natta and metallocene catalyzed isotactic polypropylene. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 3050–3064. [Google Scholar] [CrossRef]
- Pérez, E.; Cerrada, M.L.; Benavente, R.; Gómez-Elvira, J.M. Enhancing the formation of the new trigonal polymorph in isotactic propene-1-pentene copolymers: Determination of the X-ray crystallinity. Macromol. Res. 2011, 19, 1179–1185. [Google Scholar] [CrossRef]










| Sample | Reaction Time (min) | Average Activity (Kg·mol−1Zr·h−1) | Mw (g/mol) | Mw/Mn | SBA-15 Content (wt.%) |
|---|---|---|---|---|---|
| iPPhomL | 10 | 13,500 | 42,000 | 2.50 | 0 |
| iPPSBA3L | 137 | 3300 | 49,600 | 1.82 | 3.2 |
| iPPSBA6L | 10 | 14,200 | 45,700 | 1.88 | 6.1 |
| iPPSBA10L | 20 | 4400 | 9.9 | ||
| iPPSBA13L | 11 | 8200 | 43,400 | 1.91 | 12.5 |
| iPPSBA82L | 1 | 15,900 | - | - | 82.0 |
| iPPhomH | 60 | 780 | 112,100 | 1.94 | 0 |
| iPPSBA9H | 100 | 990 | 168,531 | 4.42 | 9.0 |
| iPPSBA14H | 120 | 900 | 162,343 | 4.81 | 14.0 |
| iPPSBA23H | 30 | 1800 | 157,908 | 4.45 | 23.4 |
| iPPSBA29H | 140 | 300 | - | - | 28.9 |
| Sample | SBA-15 Content (wt.%) | mmmm (mol%) | mm (mol%) | mr (mol%) | rr (mol%) | Regio-Defects (mol%) |
|---|---|---|---|---|---|---|
| iPPhomL | 0 | 78.6 | 87.0 | 8.5 | 4.4 | 1.7 |
| iPPSBA3L | 3.2 | 81.1 | 89.2 | 7.7 | 3.1 | 1.3 |
| iPPSBA6L | 6.1 | 79.8 | 88.4 | 8.1 | 3.4 | 0.8 |
| iPPSBA10L | 9.9 | 77.9 | 87.1 | 9.1 | 3.8 | 0.8 |
| iPPSBA13L | 12.5 | 81.3 | 89.2 | 7.4 | 3.4 | 1.5 |
| iPPSBA82L | 82.0 | - | - | - | - | - |
| iPPhomH | 0 | 82.7 | 89.5 | 6.7 | 3.1 | 0.6 |
| iPPSBA9H | 9.0 | 85.4 | 91.1 | 5.6 | 2.5 | 0.8 |
| iPPSBA14H | 14.0 | 88.1 | 93.8 | 4.5 | 1.7 | 0.0 |
| iPPSBA23H | 23.4 | 83.3 | 90.1 | 5.9 | 3.3 | 0.6 |
| iPPSBA29H | 28.9 | - | - | - | - | - |
| Sample | SBA-15 Content (wt.%) | ΔHmNORM (J/g) | fcNORMm (%) | Tm (°C) | ΔHCNORM (J/g) | fcNORMC (%) | Tc (°C) |
|---|---|---|---|---|---|---|---|
| iPPhomL | 0 | 90 | 56 | 135.5 | 85 | 53 | 102.5 |
| iPPSBA3L | 3.2 | 93 | 58 | 136.0 | 90 | 56 | 103.0 |
| iPPSBA6L | 6.1 | 96 | 60 | 136.0 | 87 | 55 | 104.5 |
| iPPSBA10L | 9.9 | 103 | 64 | 136.5 | 91 | 57 | 105.0 |
| iPPSBA13L | 12.5 | 100 | 62 | 136.5 | 91 | 57 | 105.0 |
| iPPhomH | 0 | 93 | 58 | 148.0 | 96 | 60 | 113.0 |
| iPPSBA9H | 9.0 | 92 | 58 | 148.5 | 88 | 55 | 112.0 |
| iPPSBA14H | 14.0 | 90 | 56 | 148.5 | 85 | 53 | 112.5 |
| iPPSBA23H | 23.4 | 86 | 55 | 148.5 | 80 | 50 | 114.0 |
| iPPSBA29H | 28.9 | 92 | 58 | 148.5 | 78 | 49 | 114.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barranco-García, R.; García-Peñas, A.; Blázquez-Blázquez, E.; Ressia, J.A.; Quinzani, L.M.; Vallés, E.M.; Gómez-Elvira, J.M.; Pérez, E.; Cerrada, M.L. Polypropylene Nanocomposites Attained by In Situ Polymerization Using SBA-15 Particles as Support for Metallocene Catalysts: Effect of Molecular Weight and Tacticity on Crystalline Details, Phase Transitions and Rheological Behavior. Molecules 2023, 28, 4261. https://doi.org/10.3390/molecules28114261
Barranco-García R, García-Peñas A, Blázquez-Blázquez E, Ressia JA, Quinzani LM, Vallés EM, Gómez-Elvira JM, Pérez E, Cerrada ML. Polypropylene Nanocomposites Attained by In Situ Polymerization Using SBA-15 Particles as Support for Metallocene Catalysts: Effect of Molecular Weight and Tacticity on Crystalline Details, Phase Transitions and Rheological Behavior. Molecules. 2023; 28(11):4261. https://doi.org/10.3390/molecules28114261
Chicago/Turabian StyleBarranco-García, Rosa, Alberto García-Peñas, Enrique Blázquez-Blázquez, Jorge A. Ressia, Lidia M. Quinzani, Enrique M. Vallés, José M. Gómez-Elvira, Ernesto Pérez, and María L. Cerrada. 2023. "Polypropylene Nanocomposites Attained by In Situ Polymerization Using SBA-15 Particles as Support for Metallocene Catalysts: Effect of Molecular Weight and Tacticity on Crystalline Details, Phase Transitions and Rheological Behavior" Molecules 28, no. 11: 4261. https://doi.org/10.3390/molecules28114261