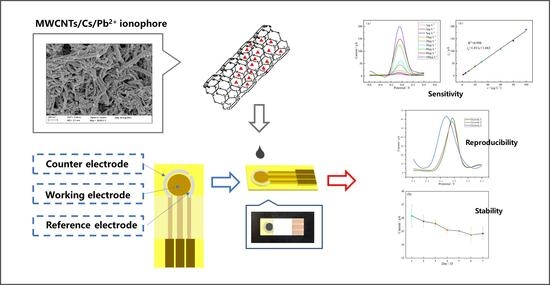
A Planar Disk Electrode Chip Based on MWCNT/CS/Pb2+ Ionophore IV Nanomaterial Membrane for Trace Level Pb2+ Detection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphologies and Feasibility Investigation of the Modified Electrodes
- is the peak current in amperes;
- A is the effective area of the electrode in cm2;
- D is the diffusion coefficient in cm2 · s−1;
- n is the number of electrons involved in the reaction;
- v is the scan rate in V · s−1;
- c is the concentration in mol · cm−3.
2.2. Optimization of Parameters
2.2.1. Optimization of the Deposition Potential
2.2.2. Optimization of the pH Value
2.2.3. Optimization of Deposition Time
2.2.4. Optimization of the Concentrations of MWCNTs, CS and Pb Ionophore IV
2.2.5. Orthogonal Experiment
2.3. Detection of Pb
2.4. Stability and Selectivity
2.5. Real Sample Detection
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Electrochemical Measurements
3.3. Electrode and Microcavity Fabrication
3.4. Preparation of a MWCNT/CS/Pb Ionophore IV Nanocomposite Membrane
3.5. Modification of the MWCNT/CS/Pb Ionophore IV/Au Electrode
3.6. Preparation of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jiang, Y.; Chao, S.; Liu, J.; Yang, Y.; Chen, Y.; Zhang, A.; Cao, H. Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 2017, 168, 1658–1668. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Q.; Nie, X.; Ren, H.; Shen, Z.; Xie, X.; Yang, Y. Heavy metal contamination in the cultivated oyster Crassostrea rivularis and associated health risks from a typical mariculture zone in the South China Sea. Bull. Environ. Contam. Toxicol. 2018, 101, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, C.; Wang, S.; Liu, Q.; Feng, H.; Ma, X.; Guo, J. Electrochemical microfluidics techniques for heavy metal ion detection. Analyst 2018, 143, 4230–4246. [Google Scholar] [CrossRef]
- Fernandez-Luqueno, F.; Lopez-Valdez, F.; Gamero-Melo, P.; Luna-Suarez, S.; Aguilera-Gonzalez, E.N.; Martínez, A.I.; García-Guillermo, M.; Hernandez-Martinez, G.; Herrera-Mendoza, R.; Álvarez-Garza, M.A.; et al. Heavy metal pollution in drinking water-a global risk for human health: A review. Afr. J. Environ. Sci. Technol. 2013, 7, 567–584. [Google Scholar]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Tahir, H.E.; Zou, X. Determinations of trace lead in various natural samples by a novel active microband-electrode probe. Chem. Eng. J. 2017, 309, 305–312. [Google Scholar] [CrossRef]
- Varney, M.W.; Aslam, D.M.; Janoudi, A.; Chan, H.Y.; Wang, D.H. Polycrystalline-diamond MEMS biosensors including neural microelectrode-arrays. Biosensors 2011, 1, 118–133. [Google Scholar] [CrossRef]
- Tang, L.J.; Wang, M.H.; Tian, H.C.; Kang, X.Y.; Hong, W.; Liu, J.Q. Progress in research of flexible MEMS microelectrodes for neural interface. Micromachines 2017, 8, 281. [Google Scholar] [CrossRef]
- Dresselhaus, M. New tricks with nanotubes. Nature 1998, 391, 19–20. [Google Scholar] [CrossRef]
- de Heer, W.A.; Bonard, J.M.; Fauth, K.; Châtelain, A.; Ugarte, D.; Forró, L. Electron Field Emitters Based on Carbon Nanotube Films; Wiley Online Library: Hoboken, NJ, USA, 1997. [Google Scholar]
- Tans, S.J.; Verschueren, A.R.; Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature 1998, 393, 49–52. [Google Scholar] [CrossRef]
- Dresselhaus, M. Down the straight and narrow. Nature 1992, 358, 195–196. [Google Scholar] [CrossRef]
- Luo, H.; Shi, Z.; Li, N.; Gu, Z.; Zhuang, Q. Investigation of the electrochemical and electrocatalytic behavior of single-wall carbon nanotube film on a glassy carbon electrode. Anal. Chem. 2001, 73, 915–920. [Google Scholar] [CrossRef]
- Wu, F.H.; Zhao, G.C.; Wei, X.W. Electrocatalytic oxidation of nitric oxide at multi-walled carbon nanotubes modified electrode. Electrochem. Commun. 2002, 4, 690–694. [Google Scholar] [CrossRef]
- Price, G.J.; Bibi, S.; Imran, Z.; Nawaz, M.; Yasin, T.; Farooq, A. Comparison of the effects of gamma or sonochemical irradiation of carbon nanotubes and the influence on the mechanical and dielectric properties of chitosan nanocomposites. Ultrason. Sonochem. 2019, 54, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.F.; Han, X.J.; Liu, Y.Q. SWASV performance toward heavy metal ions based on a high-activity and simple magnetic chitosan sensing nanomaterials. J. Alloys Compd. 2016, 684, 1–7. [Google Scholar] [CrossRef]
- Chen, Z.; Pourabedi, Z.; Hibbert, D.B. Stripping Voltammetry of Pb (II), Cu (II), and Hg (II) at a Nafion-Coated Glassy Carbon Electrode Modified by Neutral Ionophores. Electroanalysis 1999, 11, 964–968. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, X.; Zhen, Q.; He, Y.; Chen, X.; Lyu, W.; Han, R.; Ding, M. An electrochemical sensor for indole in plasma based on MWCNTs-chitosan modified screen-printed carbon electrode. Biosens. Bioelectron. 2017, 98, 392–397. [Google Scholar] [CrossRef]
- Yan, Y.; Qiu, C.; Qu, W.; Zhuang, Y.; Wang, C. Detection of BaP in seawater based on multi-walled carbon nanotubes composites immunosenor. Front. Chem. 2022, 10, 950854. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Xue, Q.; Wang, R.; Liu, Z.; Liu, Y.; He, L.; Liu, F.; Xie, H. Development of a novel sensor based on Bi2O3 and carbonized UIO-66-NH2 nanocomposite for efficient detection of Pb(II) ion in water environment. Appl. Surf. Sci. 2023, 616. [Google Scholar] [CrossRef]
- Wardak, C. 1-Hexyl-3-methylimidazolium hexafluorophosphate as new component of polymeric membrane of lead ion-selective electrode. Desalin. Water Treat. 2013, 51, 658–664. [Google Scholar] [CrossRef]
- Hui, W.; Yang, L.; Gang, L. Reusable resistive aptasensor for Pb(II) based on the Pb(II)-induced despiralization of a DNA duplex and formation of a G-quadruplex. Microchim. Acta 2018, 185, 142. [Google Scholar]
- Ding, J.; Liu, Y.; Zhang, D.; Yu, M.; Zhan, X.; Zhang, D.; Zhou, P. An electrochemical aptasensor based on gold@polypyrrole composites for detection of lead ions. Microchim. Acta 2018, 185, 545. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Y.; Zhang, D.; Yu, M.; Zhan, X.; Zhang, D.; Zhou, P. An electrochemical aptasensor for detection of lead ions using a screen-printed carbon electrode modified with Au/polypyrrole composites and toluidine blue. Anal. Methods 2019, 11, 4274–4279. [Google Scholar] [CrossRef]
- Molinero-Abad, B.; Izquierdo, D.; Pérez, L.; Escudero, I.; Arcos-Martínez, M.J. Comparison of backing materials of screen printed electrochemical sensors for direct determination of the sub-nanomolar concentration of lead in seawater. Talanta 2018, 182, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sankar, S.; Jin, C.; Sejoon, L.; Deuk, Y.K.; Ramalingam, M. A polyrutin/AgNPs coated GCE for simultaneous anodic stripping voltammetric determination of Pb(II) and Cd(II)ions in environmental samples. Colloids Surf. Physicochem. Eng. Asp. 2022, 648, 129082. [Google Scholar]
- Cecylia, W.; Klaudia, M.; Beata, P.B.; Malgorzata, G. Improved Lead Sensing Using a Solid-Contact Ion-Selective Electrode with Polymeric Membrane Modified with Carbon Nanofibers and Ionic Liquid Nanocomposite. Materials 2023, 16, 1003. [Google Scholar]
- Pei, J.; Yu, X.; Zhang, Z.; Zhang, J.; Wei, S.; Boukherroub, R. In-situ graphene modified self-supported boron-doped diamond electrode for Pb(II) electrochemical detection in seawater. Appl. Surf. Sci. 2020, 527, 146761. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Wu, Q.; Batley, G.E. Determination of sub-nanomolar concentrations of lead in sea water by adsorptive stripping voltammetry with xylenol orange. Anal. Chim. Acta 1995, 309, 95–101. [Google Scholar] [CrossRef]
- Huang, J.Y.; Bao, T.; Hu, T.X.; Wen, W.; Zhang, X.H.; Wang, S.F. Voltammetric determination of levofloxacin using a glassy carbon electrode modified with poly (o-aminophenol) and graphene quantum dots. Microchim. Acta 2017, 184, 127–135. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G. Sensitive determination of trace Cd (ii) and Pb (ii) in soil by an improved stripping voltammetry method using two different in situ plated bismuth-film electrodes based on a novel electrochemical measurement system. RSC Adv. 2018, 8, 5079–5089. [Google Scholar] [CrossRef]
- Anastasiadou, Z.D.; Jannakoudakis, P.D.; Girousi, S.T. Square wave anodic stripping voltammetry determination of eco-toxic metals in samples of biological and environmental importance. Cent. Eur. J. Chem. 2010, 8, 999–1008. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, X.; Ma, Q.; Tang, B.; Lu, Z.; Zhang, J.; Mo, G.; Ye, J.; Ye, J. A sensitive electrochemical sensor for simultaneous voltammetric sensing of cadmium and lead based on Fe3O4/multiwalled carbon nanotube/laser scribed graphene composites functionalized with chitosan modified electrode. Mater. Chem. Phys. 2019, 238, 121877. [Google Scholar] [CrossRef]
- Shoub, S.A.B.; Yusof, N.A.; Hajian, R. Gold nanoparticles/ionophore-modified screen-printed electrode for detection of Pb (II) in river water using linear sweep anodic stripping voltammetry. Sens. Mater. 2017, 29, 555–565. [Google Scholar]
- Pan, D.; Wang, Y.; Chen, Z.; Lou, T.; Qin, W. Nanomaterial/ionophore-based electrode for anodic stripping voltammetric determination of lead: An electrochemical sensing platform toward heavy metals. Anal. Chem. 2009, 81, 5088–5094. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jang, A. Measurement of Lead in Seawater using Gold Nanoparticles Modified Screen Printed Carbon Electrode. J. Coast. Res. 2017, 79, 45–49. [Google Scholar] [CrossRef]
- Tran, H.D.; Le, A.H.Q.; Tran, U.P.N. Preparation of Electrode Material Based to Bismuth Oxide-Attached Multiwalled Carbon Nanotubes for Lead (II) Ion Determination. J. Nanomater. 2021, 1687–4110. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Fu, Y.; Zhuang, Z.; Liu, Z. Indium-doped mesoporous Bi2S3-based electrochemical interface for highly sensitive detection of Pb(II). Microchem. J. 2021, 166, 106251. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, W.; Shen, Z.; Sun, S.; Dai, H.; Ma, H.; Lin, M. Sensitive and selective detection of Pb (II) and Cu (II) using a metal-organic framework/polypyrrole nanocomposite functionalized electrode. Sens. Actuators B Chem. 2020, 304, 127286. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Cui, X.; Wang, F.; Ji, H.; Du, X.; Lu, X. GaOOH-modified metal-organic frameworks UiO-66-NH2: Selective and sensitive sensing four heavy-metal ions in real wastewater by electrochemical method. Talanta 2021, 234, 122679. [Google Scholar] [CrossRef]
- Zhu, X.; Tong, J.; Bian, C.; Gao, C.; Xia, S. The polypyrrole/multiwalled carbon nanotube modified au microelectrode for sensitive electrochemical detection of trace levels of Pb2+. Micromachines 2017, 8, 86. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2018, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]








| Experiments | MWCNTs /(mg · mL−1) | Lead Ionophore IV /(mg · mL−1) | CS /(%) | Peck Current /(μA) |
|---|---|---|---|---|
| Experiment 1 | 1 | 0.5 | 0.2 | 11.090 |
| Experiment 2 | 1 | 0.7 | 0.25 | 14.260 |
| Experiment 3 | 1 | 0.9 | 0.3 | 13.240 |
| Experiment 4 | 1.5 | 0.5 | 0.25 | 17.330 |
| Experiment 5 | 1.5 | 0.7 | 0.3 | 15.770 |
| Experiment 6 | 1.5 | 0.9 | 0.2 | 14.460 |
| Experiment 7 | 2 | 0.5 | 0.3 | 12.740 |
| Experiment 8 | 2 | 0.7 | 0.2 | 14.190 |
| Experiment 9 | 2 | 0.9 | 0.25 | 15.580 |
| Average | MWCNTs | Average Peck Current | Lead Ionophore IV | Average Peck Current | CS | Average Peck Current |
|---|---|---|---|---|---|---|
| /(mg · mL−1) | /(μA) | /(mg · mL−1) | /(μA) | /(%) | /(μA) | |
| Average I | 1 | 12.863 | 0.5 | 13.720 | 0.2 | 14.147 |
| Average II | 1.5 | 15.853 | 0.7 | 14.740 | 0.25 | 15.723 |
| Average III | 2 | 14.170 | 0.9 | 14.427 | 0.3 | 13.917 |
| Range R | – | 2.990 | – | 1.020 | – | 2.476 |
| Method | Linear Range | LOD | Reference |
|---|---|---|---|
| /(μg · L−1) | /(μg · L−1) | ||
| Pt/ISM (9%NC) | 5–10.000 μmol · L−1 | 3.1 μmol · L−1 | [27] |
| G/SBDD | 1–100 | 0.21 | [28] |
| AuNPs-SPCE | 2–500 | 4.4 | [37] |
| Bi2O3@CNTs/GCE | 2–40 | 3.4 | [38] |
| IDB/GCE | 20.7–207 | 3.5 | [39] |
| PPy/NH2-MIL-53/GCE | 1–400 | 0.31 | [40] |
| UIO-66-NH2/GaOOH/GCE | 114–517 | 5.8 | [41] |
| MWCNT/CS/Pb/Au | 1–100 | 0.08 | this work |
| Seawater | Found | ICP-MS | Added | Found | Recovery | RSD (%) |
|---|---|---|---|---|---|---|
| Samples | /(μg · L) | /(μg · L) | /(μg · L) | /(μg · L) | /(%) | (n = 3) |
| 1.86 ± 0.13 | 1.8 | 0 | – | – | – | |
| Sample 1 | – | – | 5 | 6.70 ± 0.20 | 96.8% | 3.1% |
| – | – | 10 | 11.33 ± 0.50 | 94.7% | 4.4% | |
| 1.14 ± 0.08 | 1.2 | – | – | – | – | |
| Sample 2 | – | – | 5 | 6.49 ± 0.29 | 107% | 4.5% |
| – | – | 10 | 10.85 ± 0.56 | 97.1% | 5.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Y.; Wang, C.; Qu, W.; Yan, Y.; Wang, P.; Qiu, C. A Planar Disk Electrode Chip Based on MWCNT/CS/Pb2+ Ionophore IV Nanomaterial Membrane for Trace Level Pb2+ Detection. Molecules 2023, 28, 4142. https://doi.org/10.3390/molecules28104142
Zhuang Y, Wang C, Qu W, Yan Y, Wang P, Qiu C. A Planar Disk Electrode Chip Based on MWCNT/CS/Pb2+ Ionophore IV Nanomaterial Membrane for Trace Level Pb2+ Detection. Molecules. 2023; 28(10):4142. https://doi.org/10.3390/molecules28104142
Chicago/Turabian StyleZhuang, Yuan, Cong Wang, Wei Qu, Yirou Yan, Ping Wang, and Chengjun Qiu. 2023. "A Planar Disk Electrode Chip Based on MWCNT/CS/Pb2+ Ionophore IV Nanomaterial Membrane for Trace Level Pb2+ Detection" Molecules 28, no. 10: 4142. https://doi.org/10.3390/molecules28104142