Physicochemical, Structural, and In Vitro Gastrointestinal Tract Release Properties of Sodium Alginate-Based Cryogel Beads Filled with Hydroxypropyl Distarch Phosphate as a Curcumin Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
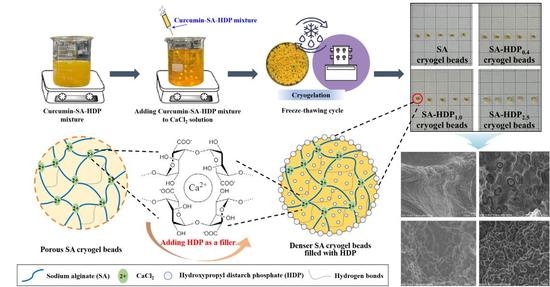
2.2. Preparation of SA-Based Cryogel Beads Filled with HDP
2.3. Scanning Electron Microscope (SEM) Analysis
2.4. X-ray Diffraction (XRD) Analysis
2.5. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis
2.6. Differential Scanning Calorimetry (DSC) Analysis
2.7. Encapsulation Efficiency of Curcumin
2.8. Swelling Ratio
2.9. In Vitro Gastrointestinal Tract Release Study
2.10. Statistical Analysis
3. Results and Discussion
3.1. Scanning Electron Microscope (SEM) Images
3.2. X-ray Diffraction (XRD) Analysis
3.3. Differential Scanning Calorimetry (DSC) Analysis
3.4. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis
3.5. Encapsulation Efficiency of Curcumin
3.6. Swelling Ratio
3.7. In Vitro Gastrointestinal Tract Release Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Memic, A.; Colombani, T.; Eggermont, L.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Joshi-Navare, K.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef] [Green Version]
- Plieva, F.M.; Galaev, I.Y.; Noppe, W.; Mattiasson, B. Cryogel applications in microbiology. Trends Microbiol. 2008, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Shchekoltsova, A.O.; Sinitskaya, E.S.; Vernaya, O.I.; Nuzhdina, A.V.; Bakeeva, I.V.; Ezernitskaya, M.G.; Semenov, A.M.; Shabatina, T.I.; Melnikov, M.Y. Influence of succinylation of a wide-pore albumin cryogels on their properties, structure, biodegradability, and release dynamics of dioxidine loaded in such spongy carriers. Int. J. Biol. Macromol. 2020, 160, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Yang, H.; Shan, Z.; Huang, K.; Zhang, M.; Xia, X. Dual-modified starch nanospheres encapsulated with curcumin by self-assembly: Structure, physicochemical properties and anti-inflammatory activity. Int. J. Biol. Macromol. 2021, 191, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Bukhari, S.N.A.; Lajis, N.H.; Abas, F.; Wai, L.K.; Jasamai, M. Effects of diarylpentanoid analogues of curcumin on chemiluminescence and chemotactic activities of phagocytes. J. Pharm. Pharmacol. 2012, 64, 404–412. [Google Scholar] [CrossRef]
- Liu, K.; Huang, R.L.; Zha, X.Q.; Li, Q.M.; Pan, L.H.; Luo, J.P. Encapsulation and sustained release of curcumin by a composite hydrogel of lotus root amylopectin and chitosan. Carbohydr. Polym. 2020, 232, 115810. [Google Scholar] [CrossRef]
- Yun, J.M.; Jialal, I.; Devaraj, S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 2011, 22, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, gut microbiota, and neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Sanidad, K.Z.; Sukamtoh, E.; Zhang, G. Potential roles of chemical degradation in the biological activities of curcumin. Food Funct. 2017, 8, 907–914. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Ułamek-Kozioł, M. Mutual two-way interactions of curcumin and gut microbiota. Int. J. Mol. Sci. 2020, 21, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zam, W. Gut microbiota as a prospective therapeutic target for curcumin: A review of mutual influence. J. Nutr. Metab. 2018, 2018, 1367984. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Vazquez, G.; Lobato-Calleros, C.; Escalona-Buendia, H.; Chavez, G.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of the weight ratio of alginate-modified tapioca starch on the physicochemical properties and release kinetics of chlorogenic acid containing beads. Food Hydrocoll. 2015, 48, 301–311. [Google Scholar] [CrossRef]
- Bušić, A.; Belščak-Cvitanović, A.; Vojvodić Cebin, A.; Karlović, S.; Kovač, V.; Špoljarić, I.; Mršić, G.; Komes, D. Structuring new alginate network aimed for delivery of dandelion (Taraxacum officinale L.) polyphenols using ionic gelation and new filler materials. Food Res. Int. 2018, 111, 244–255. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of oxidised starch (E 1404), monostarch phosphate (E 1410), distarch phosphate (E 1412), phosphated distarch phosphate (E 1413), acetylated distarch phosphate (E 1414), acetylated starch (E 1420), acetylated distarch adipate (E 1422), hydroxypropyl starch (E 1440), hydroxypropyl distarch phosphate (E 1442), starch sodium octenyl succinate (E 1450), acetylated oxidised starch (E 1451) and starch aluminium octenyl succinate (E 1452) as food additives. EFSA J. 2017, 15(10), e04911. [Google Scholar]
- de Oliveira Cardoso, V.M.; Cury, B.S.F.; Evangelista, R.C.; Gremião, M.P.D. Development and characterization of cross-linked gellan gum and retrograded starch blend hydrogels for drug delivery applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 317–333. [Google Scholar] [CrossRef] [Green Version]
- Zafeiri, I.; Beri, A.; Linter, B.; Norton, I. Mechanical properties of starch-filled alginate gel particles. Carbohydr. Polym. 2021, 255, 117373. [Google Scholar] [CrossRef]
- López Córdoba, A.; Deladino, L.; Martino, M. Effect of starch filler on calcium-alginate hydrogels loaded with yerba mate antioxidants. Carbohydr. Polym. 2013, 95, 315–323. [Google Scholar] [CrossRef]
- Li, Q.; Duan, M.; Hou, D.; Chen, X.; Shi, J.; Zhou, W. Fabrication and characterization of Ca(II)-alginate-based beads combined with different polysaccharides as vehicles for delivery, release and storage of tea polyphenols. Food Hydrocoll. 2021, 112, 106274. [Google Scholar] [CrossRef]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Freeze-thaw as a route to build manageable polysaccharide cryogel for deep cleaning of crystal violet. Chem. Eng. J. 2020, 396, 125354. [Google Scholar] [CrossRef]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core–shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein–pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef]
- Sáez, P.; Dinu, I.A.; Rodríguez, A.; Gómez, J.M.; Lazar, M.M.; Rossini, D.; Dinu, M.V. Composite cryo-beads of chitosan reinforced with natural zeolites with remarkable elasticity and switching on/off selectivity for heavy metal ions. Int. J. Biol. Macromol. 2020, 164, 2432–2449. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; McClements, D.J. Vitamin E bioaccessibility: Influence of carrier oil type on digestion and release of emulsified α-tocopherol acetate. Food Chem. 2013, 141, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yu, S.; Lin, D.; Wu, Z.; Xu, J.; Zhang, J.; Ding, Z.; Miao, Y.; Liu, T.; Chen, T.; et al. Preparation, characterization, and release behavior of doxorubicin hydrochloride from dual cross-linked chitosan/alginate hydrogel beads. ACS Appl. Bio Mater. 2020, 3, 3057–3065. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.-S.; Wong, S.-L.; Lee, P.-P.; Lee, J.-S.; Ti, T.B.; Zhang, Z.; Poncelet, D.; Ravindra, P.; Phan, S.-H.; Yim, Z.-H. Effects of starch filler on the physical properties of lyophilized calcium–alginate beads and the viability of encapsulated cells. Carbohydr. Polym. 2011, 83, 225–232. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Z.G.; Xiao, Z.G. Preparation, physicochemical characterization and in vitro release behavior of resveratrol-loaded oxidized gellan gum/resistant starch hydrogel beads. Carbohydr. Polym. 2021, 260, 117794. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Hu, Y.; Tiwari, J.K.; Velikov, K.P. Synthesis and characterisation of zein-curcumin colloidal particles. Soft Matter. 2010, 6, 6192–6199. [Google Scholar] [CrossRef]
- Kazeminejadfard, F.; Hojjati, M.R. Preparation of superabsorbent composite based on acrylic acid-hydroxypropyl distarch phosphate and clinoptilolite for agricultural applications. J. Appl. Polym. Sci. 2019, 136, 47365. [Google Scholar] [CrossRef]
- Rathna, G.V.N.; Birajdar, M.S.; Bhagwani, M.; Paul, V.L. Studies on fabrication, characterization, and metal extraction using metal chelating nonwoven nanofiber mats of poly(vinyl alcohol) and sodium alginate blends. Polym. Eng. Sci. 2013, 53, 321–333. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Fareez, I.M.; Lim, S.M.; Mishra, R.K.; Ramasamy, K. Chitosan coated alginate–xanthan gum bead enhanced pH and thermotolerance of Lactobacillus plantarum LAB12. Int. J. Biol. Macromol. 2015, 72, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Rather, S.A.; Akhter, R.; Masoodi, F.A.; Gani, A.; Wani, S.M. Effect of double alginate microencapsulation on in vitro digestibility and thermal tolerance of Lactobacillus plantarum NCDC201 and L. casei NCDC297. LWT-Food Sci. Technol. 2017, 83, 50–58. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; German, J.B.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Aliabadi, S.; Khaksar, R. Preparation and characterization of alginate and alginate-resistant starch microparticles containing nisin. Carbohydr. Polym. 2014, 103, 573–580. [Google Scholar] [CrossRef]
- Charmi, J.; Nosrati, H.; Mostafavi Amjad, J.; Mohammadkhani, R.; Danafar, H. Polyethylene glycol (PEG) decorated graphene oxide nanosheets for controlled release curcumin delivery. Heliyon 2019, 5, e01466. [Google Scholar] [CrossRef] [Green Version]
- Park, H.R.; Rho, S.J.; Kim, Y.R. Solubility, stability, and bioaccessibility improvement of curcumin encapsulated using 4-α-glucanotransferase-modified rice starch with reversible pH-induced aggregation property. Food Hydrocoll. 2019, 95, 19–32. [Google Scholar] [CrossRef]
- Priya Sharma, A.K.; Kaith, B.S.; Vipula Chandel, K.; Singh, A.; Isha. Chemically modified chitosan-sodium alginate as chemo-sensor adsorbent for the detection of picric acid and removal of biebrich scarlet. Int. J. Biol. Macromol. 2020, 147, 582–594. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, Y.; Han, C.; Zhang, H.; Tian, Y. Encapsulation of curcumin in zein/ caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocoll. 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Lin, D.; Zhou, W.; He, Q.; Xing, B.; Wu, Z.; Chen, H.; Wu, D.; Zhang, Q.; Qin, W. Study on preparation and physicochemical properties of hydroxypropylated starch with different degree of substitution under microwave assistance. Int. J. Biol. Macromol. 2019, 125, 290–299. [Google Scholar] [CrossRef]
- Xie, W.; Shao, L. Phosphorylation of corn starch in an ionic liquid. Starch/Staerke 2009, 61, 702–708. [Google Scholar] [CrossRef]
- Deeyai, P.; Suphantharika, M.; Wongsagonsup, R.; Dangtip, S. Characterization of modified tapioca starch in atmospheric argon plasma under diverse humidity by FTIR spectroscopy. Chin. Phys. Lett. 2013, 30, 181031–181034. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z. Fabrication of curcumin-loaded whey protein microgels: Structural properties, antioxidant activity, and in vitro release behavior. LWT 2019, 103, 94–100. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. pH-sensitive sodium alginate/poly(vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Yu, X.; Yan, G.; Tang, X.; Sun, Y.; Zeng, X.; Lin, L. Cellulose nanocrystalline hydrogel based on a choline chloride deep eutectic solvent as wearable strain sensor for human motion. Carbohydr. Polym. 2021, 255, 117443. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Huang, R.M.; Wu, R.Q.; Wei, Y.S.; Zong, M.H.; Linhardt, R.J.; Wu, H. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 2020, 310, 125977. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, R.; Belscak-Cvitanovic, A.; Manojlovic, V.; Komes, D.; Nedovic, V.; Bugarski, B. Encapsulation of thyme (Thymus serpyllum L.) aqueous extract in calcium alginate beads. J. Sci. Food Agric. 2012, 92, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Dafe, A.; Etemadi, H.; Dilmaghani, A.; Mahdavinia, G.R. Investigation of pectin/starch hydrogel as a carrier for oral delivery of probiotic bacteria. Int. J. Biol. Macromol. 2017, 97, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Dordević, V.; Karlović, S.; Pavlović, V.; Komes, D.; Ježek, D.; Bugarski, B.; Nedović, V. Protein-reinforced and chitosan-pectin coated alginate microparticles for delivery of flavan-3-ol antioxidants and caffeine from green tea extract. Food Hydrocoll. 2015, 51, 361–374. [Google Scholar] [CrossRef]
- Haramizu, S.; Shimotoyodome, A.; Fukuoka, D.; Murase, T.; Hase, T. Hydroxypropylated distarch phosphate versus unmodified tapioca starch: Fat oxidation and endurance in C57BL/6J mice. Eur. J. Appl. Physiol. 2012, 112, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.M.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients 2020, 13, 206. [Google Scholar] [CrossRef] [PubMed]







| Samples (1) | Encapsulation Efficiency (%) |
|---|---|
| SA cryogel beads | 31.95 ± 0.14 d(2) |
| SA-HDP0.4 cryogel beads | 45.61 ± 0.81 c |
| SA-HDP1.0 cryogel beads | 56.52 ± 0.69 b |
| SA-HDP2.5 cryogel beads | 76.66 ± 0.98 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, E.C.; Chang, Y.H. Physicochemical, Structural, and In Vitro Gastrointestinal Tract Release Properties of Sodium Alginate-Based Cryogel Beads Filled with Hydroxypropyl Distarch Phosphate as a Curcumin Delivery System. Molecules 2023, 28, 31. https://doi.org/10.3390/molecules28010031
Moon EC, Chang YH. Physicochemical, Structural, and In Vitro Gastrointestinal Tract Release Properties of Sodium Alginate-Based Cryogel Beads Filled with Hydroxypropyl Distarch Phosphate as a Curcumin Delivery System. Molecules. 2023; 28(1):31. https://doi.org/10.3390/molecules28010031
Chicago/Turabian StyleMoon, Eun Chae, and Yoon Hyuk Chang. 2023. "Physicochemical, Structural, and In Vitro Gastrointestinal Tract Release Properties of Sodium Alginate-Based Cryogel Beads Filled with Hydroxypropyl Distarch Phosphate as a Curcumin Delivery System" Molecules 28, no. 1: 31. https://doi.org/10.3390/molecules28010031