Highly Active Small Aminated Quinolinequinones against Drug-Resistant Staphylococcus aureus and Candida albicans
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
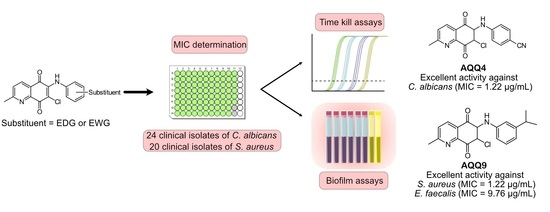
2.2. Antimicrobial Activity
2.2.1. Determination of Minimum Inhibitory Concentrations (MIC)
2.2.2. Structure-Activity Relationships (SARs) Study for Biological Evaluation
2.2.3. Time-Kill Kinetic Study
2.2.4. Evaluation of the In Vitro Antibiofilm Activity
2.2.5. In Silico Molecular Interaction Studies
3. Experimental
3.1. Chemicals and Apparatus
3.2. X-ray Diffraction Analysis
3.3. Procedure for the Synthesis of the Methyl Quinolinequinone (QQ)
6,7-Dichloro-2-methyl-5,8-quinolinequinone (QQ)
3.4. General Procedure for the Synthesis of the Aminated Quinolinequinones (AQQ1–16)
3.4.1. 7-Chloro-2-methyl-6-((2-(trifluoromethyl)phenyl)amino)-5,8-quinolinequinone (AQQ1)
3.4.2. 7-Chloro-2-methyl-6-((3-(trifluoromethyl)phenyl)amino)-5,8-quinolinequinone (AQQ2)
3.4.3. 7-Chloro-2-methyl-6-((4-(trifluoromethyl)phenyl)amino)-5,8-quinolinequinone (AQQ3)
3.4.4. 7-Chloro-6-((4-(cyano)phenyl)amino)-2-methyl-5,8-quinolinequinone (AQQ4)
3.4.5. 6-((3,5-Bis(trifluoromethyl)phenyl)amino)-7-chloro-2-methyl-5,8-quinolinequinone (AQQ5)
3.4.6. 7-Chloro-2-methyl-6-(m-tolylamino)-5,8-quinolinequinone (AQQ6)
3.4.7. 7-Chloro-2-methyl-6-(p-tolylamino)-5,8-quinolinequinone (AQQ7)
3.4.8. 7-Chloro-6-((2-isopropylphenyl)amino)-2-methyl-5,8-quinolinequinone (AQQ8)
3.4.9. 7-Chloro-6-((3-isopropylphenyl)amino)-2-methyl-5,8-quinolinequinone (AQQ9)
3.4.10. 7-Chloro-6-((4-isopropylphenyl)amino)-2-methyl-5,8-quinolinequinone (AQQ10)
3.4.11. 7-Chloro-6-((4-(diethylamino)phenyl)amino)-2-methyl-5,8-quinolinequinone (AQQ11)
3.4.12. 7-Chloro-6-((2,3-dimethylphenyl)amino)-2-methyl-5,8-quinolinequinone (AQQ12)
3.4.13. 7-Chloro-6-((2,4-dimethylphenyl)amino)-2-methyl-5,8-quinolinequinone (AQQ13)
3.4.14. 7-Chloro-6-((2,5-dimethylphenyl)amino)-2-methyl-5,8-quinolinequinone (AQQ14)
3.4.15. 7-Chloro-6-((3,4-dimethylphenyl)amino)-2-methyl-5,8-quinolinequinone (AQQ15)
3.4.16. 7-Chloro-6-((3,5-dimethylphenyl)amino)-2-methyl-5,8-quinolinequinone (AQQ16)
3.5. Biological Evaluation
3.5.1. MIC Determinations
3.5.2. Determination of Time-Kill Curves
3.5.3. Biofilm Attachment and Inhibition of Biofilm Formation Assays
3.5.4. Molecular Docking
3.5.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| CCDC | Cambridge Crystallographic Data Center |
| CLSI | Clinical and Laboratory Institute |
| EDG | Electron-donating group |
| EWG | Electron-withdrawing group |
| HPC | High-performance computing |
| HPLC | High-performance liquid chromatography |
| MIC | Minimum inhibitory concentration |
| NMR | Nuclear magnetic resonance |
| PBS | Phosphate-buffered saline |
| SAR | Structure-activity relationship |
| TKC | Time-kill curve |
| TKS | Time-kill kinetic studies |
| TLC | Thin-layer chromatography |
References
- Boudreau, M.A.; Ding, D.; Meisel, J.E.; Janardhanan, J.; Spink, E.; Peng, Z.; Qian, Y.; Yamaguchi, T.; Testero, S.A.; O’Daniel, P.I.; et al. Structure–Activity Relationship for the Oxadiazole Class of Antibacterials. ACS Med. Chem. Lett. 2020, 11, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tu, J.; Dong, G.; Wang, Y.; Sheng, C. Emerging New Targets for the Treatment of Resistant Fungal Infections. J. Med. Chem. 2018, 61, 5484–5511. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.K.; Tóth, R.; Gácser, A. Mechanisms of Pathogenic Candida Species to Evade the Host Complement Attack. Front. Cell. Infect. Microbiol. 2020, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Gullett, N.P.; Amin, A.R.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.-J.; Kucuk, O. Cancer prevention with natural compounds. Semin. Oncol. 2011, 37, 258–281. [Google Scholar] [CrossRef]
- El-Dakhakhany, M. Studies on the chemical constitution of Egyptian Nigella sativa L. seeds. Planta Med. 1963, 11, 465–470. [Google Scholar] [CrossRef]
- Sugiura, K. Antitumor activity of mitomycin C. Cancer Chemother. Rep. 1961, 13, 51–65. [Google Scholar]
- Hoyt, M.T.; Palchaudhuri, R.; Hergenrother, P.J. Cribrostatin 6 induces death in cancer cells through a reactive oxygen species (ROS)-mediated mechanism. Investig. New Drugs 2010, 29, 562–573. [Google Scholar] [CrossRef]
- Shrestha, J.P.; Subedi, Y.P.; Chen, L.; Chang, C.-W.T. A mode of action study of cationic anthraquinone analogs: A new class of highly potent anticancer agents. MedChemComm 2015, 6, 2012–2022. [Google Scholar] [CrossRef]
- Cummings, J.; Spanswick, V.J.; Tomasz, M.; Smyth, J.F. Enzymology of mitomycin C metabolic activation in tumour tissue: Implications for enzyme-directed bioreductive drug development. Biochem. Pharmacol. 1998, 56, 405–414. [Google Scholar]
- Cummings, J.; Spanswick, V.J.; Ritchie, A.A.; Smyth, J.F. Pharmacological determinants of the antitumour activity of mitomycin C—Implications for enzyme directed drug development. Ann. Oncol. 1998, 9, 134. [Google Scholar]
- Primeau, A.J.; Rendon, A.; Hedley, D.; Lilge, L.; Tannock, I.F.; Koido, S.; Hara, E.; Homma, S.; Torii, A.; Toyama, Y.; et al. The Distribution of the Anticancer Drug Doxorubicin in Relation to Blood Vessels in Solid Tumors. Clin. Cancer Res. 2005, 11, 8782–8788. [Google Scholar] [CrossRef] [Green Version]
- Patel, O.P.; Beteck, R.M.; Legoabe, L.J. Antimalarial application of quinones: A recent update. Eur. J. Med. Chem. 2021, 210, 113084. [Google Scholar] [CrossRef]
- Di Marco, N.I.; Páez, P.L.; Lucero-Estrada, C.S.M.; Pungitore, C.R. Naphthoquinones inhibit formation and viability of Yersinia enterocolitica biofilm. World J. Microbiol. Biotechnol. 2021, 37, 30. [Google Scholar] [CrossRef]
- Silakari, P.; Silakari, O.; Piplani, P. Systematic in silico Design, Synthesis, and Biological Studies of Some Novel 1,4-Benzoquinone Derivatives for the Prospective Management of Cognitive Decline. ACS Chem. Neurosci. 2021, 12, 1648–1666. [Google Scholar] [CrossRef]
- Egu, S.A.; Ibezim, A.; Onoabedje, E.A.; Okoro, U.C. Biological and in silico Evaluation of Quinolinedione and Naphthoquinone Derivatives as Potent Antibacterial Agents. ChemistrySelect 2017, 2, 9222–9226. [Google Scholar] [CrossRef]
- Keyari, C.M.; Kearns, A.K.; Duncan, N.S.; Eickholt, E.A.; Abbott, G.; Beall, H.D.; Diaz, P. Synthesis of New Quinolinequinone Derivatives and Preliminary Exploration of their Cytotoxic Properties. J. Med. Chem. 2013, 56, 3806–3819. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, K.; Espinosa-Bustos, C.; Soto-Delgado, J.; Tapia, R.A.; Varela, J.; Birriel, E.; Segura, R.; Pizarro, J.; Cerecetto, H.; González, M.; et al. New aryloxy-quinone derivatives as potential anti-Chagasic agents: Synthesis, trypanosomicidal activity, electrochemical properties, pharmacophore elucidation and 3D-QSAR analysis. RSC Adv. 2015, 5, 65153–65166. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Shim, J.-Y.; Yi, Y.-J.; Choi, I.H.; Chae, M.J.; Han, J.-Y.; Jung, O.-J. Synthesis and antifungal activity of 5,8-quinazolinedione derivatives modified at positions 6 and 7. Arch. Pharmacal Res. 2004, 27, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Alfadhli, A.; Mack, A.; Harper, L.; Berk, S.; Ritchie, C.; Barklis, E. Analysis of quinolinequinone reactivity, cytotoxicity, and anti-HIV-1 properties. Bioorg. Med. Chem. 2016, 24, 5618–5625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson-Ajinwo, O.R.; Ullah, I.; Mbye, H.; Richardson, A.; Horrocks, P.; Li, W.-W. The synthesis and evaluation of thymoquinone analogues as anti-ovarian cancer and antimalarial agents. Bioorg. Med. Chem. Lett. 2018, 28, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Prachayasittikul, V.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Novel 1,4-naphthoquinone-based sulfonamides: Synthesis, QSAR, anticancer and antimalarial studies. Eur. J. Med. Chem. 2015, 103, 446–4591. [Google Scholar] [CrossRef]
- Santoso, K.T.; Menorca, A.; Cheung, C.Y.; Cook, G.M.; Stocker, B.L.; Timmer, M.S.M. The synthesis and evaluation of quinolin-equinones as anti-mycobacterial agents. Bioorg. Med. Chem. 2019, 27, 3532–3545. [Google Scholar] [CrossRef]
- Dey, D.; Ray, R.; Hazra, B. Antitubercular and Antibacterial Activity of Quinonoid Natural Products Against Multi-Drug Resistant Clinical Isolates. Phytother. Res. 2014, 28, 1014–1021. [Google Scholar] [CrossRef]
- Nain-Perez, A.; Barbosa, L.C.; Rodríguez-Hernández, D.; Mota, Y.C.; Silva, T.F.; Ramalho, T.C.; Modolo, L.V. Antiureolytic Activity of Substituted 2,5-Diaminobenzoquinones. Chem. Biodivers. 2019, 16, e1900503. [Google Scholar] [CrossRef]
- Fröhlich, T.; Reiter, C.; Saeed, M.E.M.; Hutterer, C.; Hahn, F.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Marschall, M.; Efferth, T.; et al. Synthesis of Thymoquinone–Artemisinin Hybrids: New Potent Antileukemia, Antiviral, and Antimalarial Agents. ACS Med. Chem. Lett. 2018, 9, 534–539. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Lee, S.-Y.; Kim, N.Y.; Hong, J.A.; Yoon, J.H.; Kim, A. Synthesis and antifungal evaluation of 6-hydroxy-1H-carbazole-1,4(9H)-diones. Bioorg. Med. Chem. Lett. 2011, 21, 427–430. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Kim, A.; Im, H.A.; Kim, J.Y. Synthesis and antifungal activity of 1-thia-4b-aza-cyclopenta[b]fluorene-4,10-diones. Bioorg. Med. Chem. Lett. 2012, 22, 5777–5779. [Google Scholar] [CrossRef]
- Espinosa-Bustos, C.; Vázquez, K.; Varela, J.; Cerecetto, H.; Paulino, M.; Segura, R.; Pizarro, J.; Vera, B.; González, M.; Zarate, A.M.; et al. New aryloxy-quinone derivatives with promising activity on Trypanosoma cruzi. Arch. Pharm. 2020, 353, e1900213. [Google Scholar] [CrossRef]
- López-Lira, C.; Tapia, R.A.; Herrera, A.; Lapier, M.; Maya, J.D.; Soto-Delgado, J.; Oliver, A.G.; Lappin, A.G.; Uriarte, E. New benzimidazolequinones as trypanosomicidal agents. Bioorg. Chem. 2021, 111, 104823. [Google Scholar] [CrossRef]
- Ko, J.H.; Yeon, S.W.; Ryu, J.S.; Kim, T.-Y.; Song, E.-H.; You, H.-J.; Park, R.-E.; Ryu, C.-K. Synthesis and biological evaluation of 5-arylamino-6-chloro-1H-indazole-4,7-diones as inhibitors of protein kinase B/Akt. Bioorg. Med. Chem. Lett. 2006, 16, 6001–6005. [Google Scholar] [CrossRef]
- Chung, K.-H.; Hong, S.-Y.; You, H.-J.; Park, R.-E.; Ryu, C.-K. Synthesis and biological evaluation of 5-arylamino-1H-benzo[d]imidazole-4,7-diones as inhibitor of endothelial cell proliferation. Bioorg. Med. Chem. 2006, 14, 5795–5801. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Kang, H.-Y.; Lee, S.K.; Nam, K.A.; Hong, C.Y.; Ko, W.-G.; Lee, B.-H. 5-Arylamino-2-methyl-4,7-dioxobenzothiazoles as inhibitors of cyclin-dependent kinase 4 and cytotoxic agents. Bioorg. Med. Chem. Lett. 2000, 10, 461–464. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Lee, R.-Y.; Kim, N.Y.; Kim, Y.H.; Song, A.L. Synthesis and antifungal activity of benzo[d]oxazole-4,7-diones. Bioorg. Med. Chem. Lett. 2009, 19, 5924–5926. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Chung, K.-H.; You, H.-J.; Choi, I.H.; Chae, M.J.; Han, J.-Y.; Jung, O.-J.; Kang, S.-J.; Ryu, C.-K. Synthesis and biological evaluation of benzimidazole-4,7-diones that inhibit vascular smooth muscle cell proliferation. Bioorg. Med. Chem. Lett. 2004, 14, 3563–3566. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Delgado, V.; Sepúlveda, S.; Benites, J.; Theoduloz, C.; Calderon, P.B.; Muccioli, G.G. Synthesis and Cytotoxic Activity on Human Cancer Cells of Novel Isoquinolinequinone–Amino Acid Derivatives. Molecules 2016, 21, 1199. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.-K.; Park, R.-E.; Ma, M.-Y.; Nho, J.-H. Synthesis and antifungal activity of 6-arylamino-phthalazine-5,8-diones and 6,7-bis(arylthio)-phthalazine-5,8-diones. Bioorg. Med. Chem. Lett. 2007, 17, 2577–2580. [Google Scholar] [CrossRef]
- Chung, H.-J.; Jung, O.-J.; Chae, M.J.; Hong, S.-Y.; Chung, K.-H.; Lee, S.K.; Ryu, C.-K. Synthesis and biological evaluation of quinoxaline-5,8-diones that inhibit vascular smooth muscle cell proliferation. Bioorg. Med. Chem. Lett. 2005, 15, 3380–3384. [Google Scholar] [CrossRef]
- Yoo, H.W.; Lee, Y.S.; Suh, M.E.; Kim, D.J.; Park, S.W. Cytotoxic effects of quinoxaline derivatives on human cancer cell lines. Arch. Pharm. 1998, 331, 331–333. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Bębenek, E.; Chrobak, E.; Boryczka, S. 5,8-Quinolinedione Scaffold as a Promising Moiety of Bioactive Agents. Molecules 2019, 24, 4115. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.-K.; Lee, S.-K.; Han, J.-Y.; Jung, O.-J.; Lee, J.Y.; Jeong, S.H. Synthesis and antifungal activity of 5-arylamino-4,7-dioxobenzo[b]thiophenes. Bioorg. Med. Chem. Lett. 2005, 15, 2617–2620. [Google Scholar] [CrossRef]
- Ryu, C.K.; Kang, H.Y.; Yi, Y.J.; Shin, K.H.; Lee, B.H. Synthesis and antifungal activities of 5/6-arylamino-4,7-dioxobenzothiazoles. Bioorg. Med. Chem. Lett. 2000, 10, 1589–1591. [Google Scholar] [CrossRef]
- Choi, S.Y.; Shin, J.H.; Ryu, C.K.; Nam, K.Y.; No, K.T.; Choo, H.Y.P. The development of 3D-QSAR study and recursive par-titioning of heterocyclic quinone derivatives with antifungal activity. Bioorg. Med. Chem. 2006, 14, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Jordão, A.K.; Novais, J.; Leal, B.; Escobar, A.C.; dos Santos, H.M., Jr.; Castro, H.C.; Ferreira, V. Synthesis using microwave irradiation and antibacterial evaluation of new N,O-acetals and N,S-acetals derived from 2-amino-1,4-naphthoquinones. Eur. J. Med. Chem. 2013, 63, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Lown, J.W.; Begleiter, A.; Johnson, D.; Morgan, A.R. Studies related to antitumor antibiotics. Part V. Reactions of mitomycin C with DNA examined by ethidium fluorescence assay. Can. J. Biochem. 1976, 54, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Weinreb, S.M. Streptonigrin. Fortschr. Chem. Org. Nat./Prog. Chem. Org. Nat. Prod. 1982, 41, 77–114. [Google Scholar]
- Balitz, D.M.; Bush, J.A.; Bradner, W.T.; Doyle, T.W.; O’Herron, F.A.; Nettleton, D.E. Isolation of lavendamycin. A new antibiotic from Streptomyces lavendulae. J. Antibiot. 1982, 35, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Defant, A.; Mancini, I. Design, Synthesis and Cancer Cell Growth Inhibition Evaluation of New Aminoquinone Hybrid Mol-ecules. Molecules 2019, 24, 2224. [Google Scholar] [CrossRef] [Green Version]
- Egu, S.A.; Okoro, U.C.; Onoabedje, E.A. New Aryl Derivatives of Quinolinedione and Related Heterocyclic Compounds. J. Heterocycl. Chem. 2017, 54, 1572–1577. [Google Scholar] [CrossRef]
- Patani, G.A.; LaVoie, E.J. Bioisosterism: A Rational Approach in Drug Design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Meanwell, N.A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef]
- Ibis, C.; Tuyun, A.F.; Ozsoy-Gunes, Z.; Bahar, H.; Stasevych, M.V.; Musyanovych, R.Y.; Komarovska-Porokhnyavets, O.; Novikov, V. Synthesis and biological evaluation of novel nitrogen- and sulfur-containing hetero-1,4-naphthoquinones as potent antifungal and antibacterial agents. Eur. J. Med. Chem. 2011, 46, 5861–5867. [Google Scholar] [CrossRef]
- Tuyun, A.F.; Bayrak, N.N.; Yildirim, H.; Onul, N.; Kara, E.M.; Celik, B.O. Synthesis and In Vitro Biological Evaluation of Aminonaphthoquinones and Benzo[b]phenazine-6,11-dione Derivatives as Potential Antibacterial and Antifungal Compounds. J. Chem. 2015, 2015, 645902. [Google Scholar] [CrossRef] [Green Version]
- Yıldırım, H.; Bayrak, N.; Tuyun, A.F.; Kara, E.M.; Çelik, B.; Gupta, G.K. 2,3-Disubstituted-1,4-naphthoquinones containing an arylamine with trifluoromethyl group: Synthesis, biological evaluation, and computational study. RSC Adv. 2017, 7, 25753–25764. [Google Scholar] [CrossRef] [Green Version]
- Tuyun, A.F.; Yıldız, M.; Bayrak, N.; Yıldırım, H.; Kara, E.M.; Jannuzzi, A.T.; Celik, B.O. Discovery of a new family of heterocyclic amine linked plastoquinone analogs for antimicrobial evaluation. Drug Dev. Res. 2019, 80, 1098–1109. [Google Scholar] [CrossRef]
- Kara, E.M.; Bayrak, N.; Yildirim, H.; Yildiz, M.; Celik, B.O.; Tuyun, A.F. Chlorinated plastoquinone analogs that inhibit Staphy-lococcus epidermidis and Candida albicans growth. Folia Microbiol. 2020, 65, 785–795. [Google Scholar] [CrossRef]
- Mataracı-Kara, E.; Bayrak, N.; Yıldırım, H.; Yıldız, M.; Ataman, M.; Ozbek-Celik, B.; Tuyun, A.F. Plastoquinone analogs: A po-tential antimicrobial lead structure intensely suppressing Staphylococcus epidermidis and Candida albicans growth. Med. Chem. Res. 2021, 30, 1728–1737. [Google Scholar] [CrossRef]
- Yıldız, M.; Bayrak, N.; Yıldırım, H.; Mataracı-Kara, E.; Shilkar, D.; Jayaprakash, V.; Tuyun, A.F. Exploration of brominated Plastoquinone analogs: Discovery and structure-activity relationships of small antimicrobial lead molecules. Bioorg. Chem. 2021, 116, 105316. [Google Scholar] [CrossRef]
- Bayrak, N.; Yıldız, M.; Yıldırım, H.; Kara, E.M.; Celik, B.O.; Tuyun, A.F. Brominated plastoquinone analogs: Synthesis, structural characterization, and biological evaluation. J. Mol. Struct. 2020, 1219, 128560. [Google Scholar] [CrossRef]
- Mataraci-Kara, E.; Bayrak, N.; Yildiz, M.; Yildirim, H.; Ozbek-Celik, B.; Tuyun, A.F. Discovery and structure-activity relationships of the quinolinequinones: Promising antimicrobial agents and mode of action evaluation. Drug Dev. Res. 2020, 1219, 128560. [Google Scholar]
- Bayrak, N.; Yıldız, M.; Yıldırım, H.; Mataracı-Kara, E.; Tuyun, A.F. Novel plastoquinone analogs containing benzocaine and its analogs: Structure-based design, synthesis, and structural characterization. Res. Chem. Intermed. 2021, 47, 2125–2141. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Clemons, W.M. Structure of the twin-arginine signal-binding protein DmsD fromEscherichia coli. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 746–750. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Sandalova, T.; Lindqvist, Y.; Arnér, E. Crystal Structure and Catalysis of the Selenoprotein Thioredoxin Reductase 1. J. Biol. Chem. 2009, 284, 3998–4008. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, C.; Wu, M.; Tian, T.; Cheng, T.; Zhang, X.; Zang, J. Enolase binds to RnpA in competition with PNPase in Staphylococcus aureus. FEBS Lett. 2017, 591, 3523–3535. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, I.A.; Johnson, F.; Grollman, A.P. Streptonigrin. 1. Structure-Activity Relationships Among Simple Bicyclic Analogues. Rate Dependence of DNA Degradation on Quinone Reduction Potential. J. Med. Chem. 1987, 18, 1329–1340. [Google Scholar] [CrossRef]
- Yoon, E.Y.; Choi, H.Y.; Shin, K.J.; Yoo, K.H.; Chi, D.Y.; Kim, D.J. The regioselectivity in the reaction of 6,7-dihaloquinoline-5,8-diones with amine nucleophiles in various solvents. Tetrahedron Lett. 2000, 41, 7475–7480. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Park, S.-Y.; Lee, H.-J.; Suh, M.-E.; Schollmeyer, D.; Lee, C.-O. Synthesis and cytotoxicity of 6,11-Dihydro-pyrido- and 6,11-Dihydro-benzo [2,3-b]phenazine-6,11-dione derivatives. Bioorg. Med. Chem. 2003, 11, 1709–1714. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, J.S.; Park, S.-Y.; Suh, M.-E.; Kim, H.J.; Seo, E.-K.; Lee, C.-O. Synthesis and cytotoxicity evaluation of 6,11-dihydro-pyridazo- and 6,11-dihydro-pyrido[2,3-b]phenazine-6,11-diones. Bioorg. Med. Chem. 2004, 12, 1623–1628. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Choi, J.-A.; Kim, S.-H. Synthesis and antifungal evaluation of 6-(N-arylamino)-7-methylthio-5,8-quinolinediones. Arch. Pharmacal Res. 1998, 21, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Jastrzębska, M.; Bębenek, E.; Chrobak, E.; Latocha, M.; Kusz, J.; Tarnawska, D.; Boryczka, S. New Acetylenic Amine Derivatives of 5,8-Quinolinediones: Synthesis, Crystal Structure and Antiproliferative Activity. Crystals 2017, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Kara, E.M.; Celik, B.O. Investigation of the effects of various antibiotics against Klebsiella pneumoniae biofilms on in vitro catheter model. J. Chemother. 2018, 30, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Bruker. APEX2, Version 2014.1-1; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Bruker. SAINT, Version 8.34A; Bruker AXS Inc.: Madison, WI, USA, 2013. [Google Scholar]
- Bruker. SADABS, Version 2012/2; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Bruker. SHELXTL, Version 6.14; Bruker AXS Inc.: Madison, WI, USA, 2000. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute (CLSI), Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute (CLSI), Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard–Second Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1997. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef]











| Identification Code | AQQ7 | AQQ10 |
|---|---|---|
| Chemical formula | C17H13ClN2O2 | C19H17ClN2O2 |
| Formula weight (g mol−1) | 312.74 | 340.79 |
| Temperature (K) | 292(2) | 299(2) |
| Radiation λ (Å) | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Trigonal |
| Space groups, Z | P 1 21/c 1, 4 | R 3 c, 18 |
| Unit cell dimensions (Å) | a = 4.8838 (5) | a = 28.573 (6) |
| b = 25.878 (3) | b = 28.573 (6) | |
| c = 11.6611 (12) | c = 10.771 (3) | |
| α, γ = 90° | α, β = 90° | |
| β = 98.331 (2)° | γ = 120° | |
| Volume (Å3) | 1458.2 (3) | 7615.(4) |
| Crystal sizes (mm) | 0.068 × 0.146 × 0.500 | 0.038 × 0.070 × 0.434 |
| dcalc (g cm−3) | 1.425 | 1.338 |
| Absorption coefficient (mm−1) | 0.270 | 0.239 |
| Tmin, Tmax | 0.8770, 0.9820 | 0.9030, 0.9910 |
| θmax, deg | 27.48 | 25.02 |
| Goodness-of-fit on F2 | 1.014 | 1.041 |
| Index ranges | −6 ≤ h ≤ 6 | −33 ≤ h ≤ 34 |
| −33 ≤ k ≤ 33 | −33 ≤ k ≤ 34 | |
| −15 ≤ l ≤ 15 | −12 ≤ l ≤ 12 | |
| Reflections collected | 21278 | 54427 |
| Independent reflections | 3346 [R(int) = 0.0599] | 2980 [R(int) = 0.0831] |
| Final R indices [I > 2σ(I)] | 2635 data | 1930 data |
| R1 = 0.0446 | R1 = 0.0526 | |
| wR2 = 0.1219 | wR2 = 0.1344 | |
| R indices (all data) | R1 = 0.0579 | R1 = 0.0919 |
| wR2 = 0.1305 | wR2 = 0.1589 | |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3346/0/202 | 2980/1/220 |
| Largest diff. peak and hole (eÅ−3) | 0.446 and −0.277 | 0.189 and −0.144 |
| AQQs | Subseries (X) | Substituent(s) | Gram-Negative Bacteria (MIC, μg/mL) | Gram-Positive Bacteria (MIC, μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| General Formula | ID | P. aeruginosa | E. coli | K. pneumoniae | P. mirabilis | S. aureus | S. epidermidis | E. faecalis | ||
 | AQQ1 | EWG | 2-CF3 | - | - | - | - | 1250 | 1250 | - |
| AQQ2 | 3-CF3 | - | - | - | - | 625 | 78.12 | 312.50 | ||
| AQQ3 | 4-CF3 | - | 312.50 | - | 625 | 625 | 312.50 | 625 | ||
| AQQ4 | 4-CN | - | - | 625 | - | 625 | 312.50 | 625 | ||
| AQQ5 | 3,5-diCF3 | - | - | - | - | 1250 | 39.06 | - | ||
| AQQ6 | EDG | 3-CH3 | - | - | - | - | 1250 | 78.12 | 625 | |
| AQQ7 | 4-CH3 | - | 312.50 | - | - | 625 | 78.12 | 625 | ||
| AQQ8 | 2-CH(CH3)2 | - | - | - | - | 1.22 | - | 19.53 | ||
| AQQ9 | 3-CH(CH3)2 | - | - | - | - | 1.22 | - | 9.76 | ||
| AQQ10 | 4-CH(CH3)2 | - | - | - | - | 1.22 | - | 19.53 | ||
| AQQ11 | 4-N(CH2CH3)2 | - | - | - | - | 19.53 | - | 312.50 | ||
| AQQ12 | 2,3-diCH3 | - | - | - | - | 1.22 | - | 625 | ||
| AQQ13 | 2,4-diCH3 | - | - | - | - | 1.22 | - | 625 | ||
| AQQ14 | 2,5-diCH3 | - | - | - | - | 1.22 | - | 39.06 | ||
| AQQ15 | 3,4-diCH3 | - | - | - | - | 625 | - | 156.25 | ||
| AQQ16 | 3,5-diCH3 | - | - | - | - | 19.53 | - | 625 | ||
| Ciprofloxacin | 0.125 | 0.007 | 0.125 | 0.007 | 0.25 | 0.25 | 0.25 | |||
| AQQs | Subseries (X) | Substituent(s) | Fungi (MIC, μg/mL) | |||
|---|---|---|---|---|---|---|
| General Formula | ID | C. albicans | C. parapsilosis | C. tropicalis | ||
 | AQQ1 | EWG | 2-CF3 | 312.50 | 312.50 | - |
| AQQ2 | 3-CF3 | 312.50 | 312.50 | - | ||
| AQQ3 | 4-CF3 | 312.50 | 156.25 | - | ||
| AQQ4 | 4-CN | 1.22 | 2.44 | - | ||
| AQQ5 | 3,5-diCF3 | 156.25 | 312.50 | - | ||
| AQQ6 | EDG | 3-CH3 | 156.25 | 78.12 | - | |
| AQQ7 | 4-CH3 | 156.25 | 156.25 | - | ||
| AQQ8 | 2-CH(CH3)2 | 312.50 | 78.12 | 156.25 | ||
| AQQ9 | 3-CH(CH3)2 | 312.50 | 9.76 | 312.50 | ||
| AQQ10 | 4-CH(CH3)2 | - | 312.50 | - | ||
| AQQ11 | 4-N(CH2CH3)2 | 312.50 | - | 312.50 | ||
| AQQ12 | 2,3-diCH3 | 312.50 | 312.50 | - | ||
| AQQ13 | 2,4-diCH3 | 312.50 | 312.50 | - | ||
| AQQ14 | 2,5-diCH3 | 312.50 | 19.53 | 312.50 | ||
| AQQ15 | 3,4-diCH3 | - | 312.50 | - | ||
| AQQ16 | 3,5-diCH3 | - | 312.50 | - | ||
| Clotrimazole | 4.88 | - | - | |||
| Amphotericin B | - | 0.50 | 1.00 | |||
| Protein | Compound | Binding Energy (Kcal/mol) | Inhibition Constant (Ki) (in µM) | No. of H-Bonds | Amino Acids Interactions |
|---|---|---|---|---|---|
| 3CW0 | AQQ4 | −5.36 | 117.62 | 1 (LEU82) | 11 (ARG204, VAL97, PRO85, ALA81, VAL77, PHE76, LEU98, SER96, GLU95, TRP87, VAL90) |
| AQQ9 | −5.66 | 70.88 | 1 (LEU82) | 10 (VAL90, PRO85, PRO83, ARG204, LEU98, ALA81, TRP87, VAL77, TRP80, LEU82) | |
| 3EAN | AQQ4 | −7.88 | 1.69 | 2 (GLU477, TRP407) | 13 (HIS472, ILE478, PHE406, GLN494, GLY496, SER495, ASN418, GLU410, PRO408, VAL474, PRO473, LEU409, CYS475) |
| AQQ9 | −8.43 | 0.664 | 1 (TRP407) | 14 (HIS472, PRO408, LEU409, GLU477, PRO473, CYS475, GLU410, VAL474, PHE406, CYS497, SER495, GLY496, GLN494, THR412) | |
| 5XEX | AQQ4 | −8.06 | 1.24 | 2 (PRO101, PHE103) | 11 (ILE513, LYS105, ASP55, LYS108, GLY106, TYR107, PHE57, PRO104, ARG100, LYS514, ILE513) |
| AQQ9 | −7.93 | 1.53 | 1 (PHE103) | 12 (PRO104, TYR107, GLY106, ARG100, PHE57, LEU102, PRO101, GLN146, ASP516, ILE515, LYS514, LYS105) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yıldırım, H.; Bayrak, N.; Yıldız, M.; Yılmaz, F.N.; Mataracı-Kara, E.; Shilkar, D.; Jayaprakash, V.; TuYuN, A.F. Highly Active Small Aminated Quinolinequinones against Drug-Resistant Staphylococcus aureus and Candida albicans. Molecules 2022, 27, 2923. https://doi.org/10.3390/molecules27092923
Yıldırım H, Bayrak N, Yıldız M, Yılmaz FN, Mataracı-Kara E, Shilkar D, Jayaprakash V, TuYuN AF. Highly Active Small Aminated Quinolinequinones against Drug-Resistant Staphylococcus aureus and Candida albicans. Molecules. 2022; 27(9):2923. https://doi.org/10.3390/molecules27092923
Chicago/Turabian StyleYıldırım, Hatice, Nilüfer Bayrak, Mahmut Yıldız, Fatıma Nur Yılmaz, Emel Mataracı-Kara, Deepak Shilkar, Venkatesan Jayaprakash, and Amaç Fatih TuYuN. 2022. "Highly Active Small Aminated Quinolinequinones against Drug-Resistant Staphylococcus aureus and Candida albicans" Molecules 27, no. 9: 2923. https://doi.org/10.3390/molecules27092923