Effects of Coumarinyl Schiff Bases against Phytopathogenic Fungi, the Soil-Beneficial Bacteria and Entomopathogenic Nematodes: Deeper Insight into the Mechanism of Action
Abstract
:1. Introduction
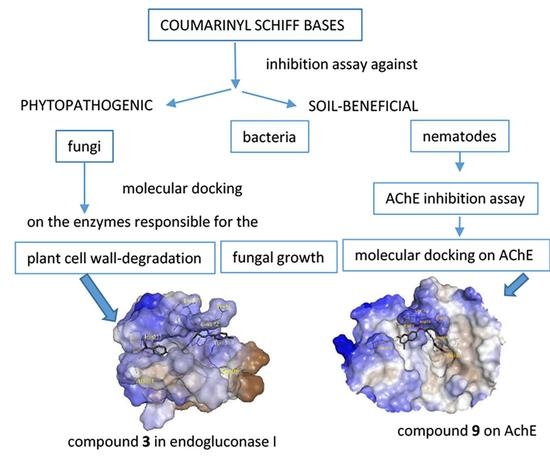
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Coumarinyl Schiff Bases
3.2. Biological Assays
3.2.1. Antifungal Assays
3.2.2. Antibacterial Assays
3.2.3. Nematicidal Assays
3.2.4. Acetylcholinesterase Inhibition Assays
3.3. Computational Methods
3.3.1. Calculation of Pesticide-Likeness Molecular Descriptors
3.3.2. Calculation of Toxicity
3.3.3. Molecular Docking
3.3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ćosić, J.; Vrandečić, K.; Svitlica, B. Fusarium vrste izolirane s pšenice i kukuruza u istočnoj Hrvatskoj. Poljoprivreda 2004, 10, 5–8. [Google Scholar]
- Ćosić, J.; Vrandečić, K.; Šimić, B.; Poštić, J.; Baličević, R. Fusarium species isolated from plant debris in Eastern Croatia. Cereal Res. Commun. 2008, 36, 55–58. [Google Scholar]
- Pogăcean, M.O.; Gavrilescu, M. Plant protection products and their sustainable and environmentally friendly use. Environ. Eng. Manag. J. 2009, 8, 608–627. [Google Scholar]
- Smith, K.; Evans, D.A.; El-Hiti, G.A. Role of modern chemistry in sustainable arable crop protection. Phil. Trans. R Soc. B 2008, 363, 623–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.K. Screening of bioactive compounds for development of new pesticides: A Mini review. Univ. J. Agric. Res. 2016, 4, 15–20. [Google Scholar] [CrossRef]
- Song, P.P.; Zhao, J.; Liu, Z.L.; Duan, Y.B.; Hou, Y.P.; Zhao, C.Q.; Wu, M.; Wei, M.; Wang, N.H.; Lv, Y.; et al. Evaluation of antifungal activities and structure–activity relationships of coumarin derivatives. Pest. Manag. Sci. 2017, 73, 94–101. [Google Scholar] [CrossRef]
- Wei, Y.; Peng, W.; Wanf, D.; Hao, S.H.; Li, W.W.; Ding, F. Design, synthesis, antifungal activity, and 3D-QSAR of coumarin derivatives. J. Pesti Sci. 2018, 43, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, P.A.; Santos Jn, M.S.; Sousa, C.S.; Góes-Neto, A.; Luz, E.D.N.; Damaceno, V.O.; Niella, A.R.R.; Filho, J.M.B.; Assis, S.A. Study of sodium 3-hydroxycoumarin as inhibitors in vitro, in vivo and in silico of Moniliophthora perniciosa fungus. Eur. J. Plant Pathol. 2019, 153, 15–27. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Y.; Li, S.; Ding, W. Resveratrol and coumarin: Novel agricultural antibacterial agent against Ralstonia solanacearum in vitro and in vivo. Molecules 2016, 21, 1501. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Ikram, M.; Baker, R.J.; Zubair, M.; Azad, E.; Min, S.; Riaz, K.; Mok, K.H.; Rehman, S.U. Synthesis, characterization, in vitro antimicrobial, and U2OS tumoricidal activities of different coumarin derivatives. Chem. Cent. J. 2013, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Dekić, B.D.; Radulović, N.S.; Dekić, V.S.; Vukićević, R.D.; Palić, R.M. Synthesis and antimicrobial activity of new 4-heteroarylamino coumarin derivatives containing nitrogen and sulfur as heteroatoms. Molecules 2010, 15, 2246–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Jin, H.; Liu, Q.; Yan, Z.; Ding, L.; Qin, B. Nematicidal metabolites from roots of Stellera chamaejasme against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Pest. Manag. Sci. 2014, 70, 827–835. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.-Z.; Sun, S.-A.; Guo, H.-R.; Qin, B. Design and synthesis of novel coumarin analogs and their nematicidal activity against five phytonematodes. Chin. Chem. Lett. 2016, 27, 375–379. [Google Scholar] [CrossRef]
- Souza, S.M.; Delle Monache, F.; Smânia, A., Jr. Antibacterial activity of coumarins. Z. Nat. 2005, 60, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Escareño-Díaz, S.; Alonso-Díaz, M.A.; Mendoza de Gives, P.; Castillo-Gallegos, E.; von Son-de Fernex, E. Anthelmintic-like activity of polyphenolic compounds and their interactions against the cattle nematode Cooperia punctata. Vet. Parasitol. 2019, 274, 108909. [Google Scholar] [CrossRef] [PubMed]
- Rastija, V.; Vrandečić, K.; Ćosić, J.; Majić, I.; Šarić, G.K.; Agić, D.; Karnaš, M.; Lončarić, M.; Molnar, M. Biological activities related to plant protection and environmental effects of coumarin derivatives: QSAR and molecular docking studies. Int. J. Mol. Sci. 2021, 22, 7283. [Google Scholar] [CrossRef]
- Güngör, Ö.; Gürkan, P. Synthesis and characterization of higher amino acid Schiff bases, as monosodium salts and neutral forms. Investigation of the intramolecular hydrogen bonding in all Schiff bases, antibacterial and antifungal activities of neutral forms. J. Mol. Struct. 2014, 1074, 62–70. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Q.; Wang, G.; Dong, F.; Zhou, H.; Zhang, J. Synthesis, characterization, and antifungal activity of novel inulin derivatives with chlorinated benzene. Carbohydr. Polym. 2014, 99, 469–473. [Google Scholar] [CrossRef]
- Wei, L.; Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Synthesis, characterization, and antifungal activity of Schiff bases of inulin bearing pyridine ring. Polymers 2019, 11, 371. [Google Scholar] [CrossRef] [Green Version]
- Tyndall, J.D.A.; Sabherwal, M.; Sagatova, A.A.; Keniya, M.V.; Negroni, J.; Wilson, R.K.; Woods, M.A.; Tietjen, K.; Monk, B.C. Structural and functional elucidation of yeast lanosterol 14-demethylase in complex with agrochemical antifungals. PLoS ONE 2016, 11, e0167485. [Google Scholar] [CrossRef] [PubMed]
- Verburg, J.G.; Huynh, Q.K. Purification and characterization of an antifungal chitinase from Arabidopsis thaliana. Plant Physiol. 1991, 95, 450–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodge, J.K.; Johnson, R.L.; Weinberg, R.A.; Gordon, J.I. Comparison of myristoyl-CoA:protein N-myristoyltransferases from three pathogenic fungi: Cryptococcus neoformans, Histoplasma capsulatum, and Candida albicans. J. BiolChem. 1994, 269, 2996–3009. [Google Scholar] [CrossRef]
- Ramos, A.M.; Gally, M.; Szapiro, G.; Itzcovich, T.; Carabajal, M.; Levin, L. In vitro growth and cell wall degrading enzyme production by Argentinean isolates of Macrophomina phaseolina, the causative agent of charcoal rot in corn. Rev. Argent Microbiol. 2016, 21, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Majić, I.; Sarajlić, A.; Lakatos, T.; Tóth, T.; Raspudić, E.; Šarić, G.K.; Laznik, Ž. Compatibility of bio-nematicide and plant stimulant of microbial origin with Heterorhabditis bacteriophora. IOBC/WPRS Bull. 2017, 129, 125–129. [Google Scholar]
- Bai, X.; Adams, B.J.; Ciche, T.A.; Clifton, S.; Gaugler, R.; Kim, K.S.; Grewal, P.S. A lover and a fighter: The genome sequence of an entomopathogenic nematode Heterorhabditis bacteriophora. PLoS ONE 2013, 8, e69618. [Google Scholar] [CrossRef] [Green Version]
- Mladenović, M.; Arsić, B.B.; Stanković, N.; Mihović, N.; Ragno, R.; Regan, A.; Miličević, J.S.; Trtić-Petrović, T.; Micić, R. The targeted pesticides as acetylcholinesterase Inhibitors: Comprehensive cross-organism molecular modelling studies performed to anticipate the pharmacology of harmfulness to humans in vitro the targeted. Molecules 2018, 23, 2192. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, J.; Shen, H.; Cheng, J.; Li, Z.; Xu, X. Synthesis, nematicidal activity and docking study of novel chromone derivatives containing substituted pyrazole. Chin. Chem. Lett. 2018, 29, 911–914. [Google Scholar] [CrossRef]
- Atwa, A.A.; Shamseldean, M.M.; Yonis, F.A. The effect of different pesticides on reproduction of entomopathogenic nematodes. Turk. J. Entomol. 2013, 37, 493–502. [Google Scholar]
- Molnar, M.; Komar, M.; Brahmbhatt, H.; Babić, J.; Jokić, S.; Rastija, V. Deep eutectic solvents as convenient media for synthesis of novel coumarinyl Schiff bases and their QSAR studies. Molecules 2017, 22, 1482. [Google Scholar] [CrossRef] [Green Version]
- Araújo, R.S.A.; Guerra, F.Q.S.; Lima, E.O.; Simone, C.A.; Tavares, J.F.; Scott, L.; Scotti, M.T.; Aquino, T.M.; Moura, R.O.; Mendonça, F.J.B., Jr.; et al. Synthesis, structure-activity relationships (SAR) and in silico studies of coumarin derivatives with antifungal activity. Int. J. Mol. Sci. 2013, 14, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, K.R. The biplot graphic display of matrices with application to principal component analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L.; Penghceng, L. Antifungal properties of Schiff bases of chitosan, N -substituted chitosan and quaternized chitosan. Carbohydr. Res. 2007, 342, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mi, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis of Schiff bases modified inulin derivatives for potential antifungal and antioxidant applications. Int. J. Biol. Macromol. 2020, 143, 714–723. [Google Scholar] [CrossRef]
- Lockhart, D.E.A.; Schuettelkopf, A.; Blair, D.E.; van Aalten, D.M.F. Screening-based discovery of Aspergillus fumigatus plant-type chitinase inhibitors. FEBS Lett. 2014, 588, 3282–3290. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Tao, Y.; Zhang, M.; Howard, M.H.; Gutteridge, S.; Ding, J. Crystal structures of Saccharomyces cerevisiae N-myristoyltransferase with bound myristoyl-CoA and inhibitors reveal the functional roles of the N-terminal region. J. Biol. Chem. 2007, 282, 22185–22194. [Google Scholar] [CrossRef] [Green Version]
- Sulzenbacher, G.; Schülein, M.; Davies, G.J. Structure of the endoglucanase I from Fusarium oxysporum: Native, cellobiose, and 3,4-epoxybutyl β-D-cellobioside-inhibited forms, at 2.3 Å resolution. Biochemistry 1997, 36, 5902–5911. [Google Scholar] [CrossRef]
- Olivieri, F.; Zanetti, E.; Oliva, C.R.; Covarrubias, A.A.; Casalongué, C.A. Characterization of an extracellular serine protease of Fusarium eumartii and its action on pathogenesis related proteins. Eur. J. Plant. Pathol. 2002, 108, 63–72. [Google Scholar] [CrossRef]
- Santen, Y.; Benen, J.A.E.; Schröter, K.H.; Kalk, K.H.; Armand, S.; Visser, J.; Dijkstra, B.W. 1.68-Å Crystal structure of endopolygalacturonase II from Aspergillus niger and identification of active site residues by site-directed mutagenesis. J. Biol. Chem. 1999, 274, 30474–30480. [Google Scholar] [CrossRef] [Green Version]
- Ammon, V.; Wyllie, T.D.; Brown, M.F. An ultrastructural investigation of pathological alterations induced by Macrophomina phaseolina (Tassi) Goid. in seedlings of soybean, Glycine max (L.). Merril. Physiol. Plant Pathol. 1974, 4, 1–4. [Google Scholar] [CrossRef]
- Jones, R.W.; Wang, H. Immunolocalization of a β-1,4-endoglucanase from Macrophomina phaseolina expressed in planta. Can. J. Microbiol. 1997, 43, 403–410. [Google Scholar] [CrossRef]
- Wang, H.; Jones, R.W. A unique endoglucanase-encoding gene cloned from the phytopathogenic gungus Macrophomina phaseolina. Appl. Environ. Microbiol. 1995, 61, 2004–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Wei, W.; Yuanyuan, P.; Fuping, L. Screening and identification of cellulase-producing strain of Fusarium oxysporum. Procedia Environ. Sci. 2012, 12, 1213–1219. [Google Scholar]
- Yuan, S.; Wu, Y.; Cosgrove, D.J. A Fungal Endoglucanase with Plant Cell Wall Extension Activity. Plant Physiol 2001, 127, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquez, N.; Giachero, M.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General characteristics of pathogenicity and methods of control. Front. Plant. Sci. 2021, 12, 634397. [Google Scholar] [CrossRef] [PubMed]
- Santhi, V.S.; Salame, L.; Muklada, H.; Azaizeh, H.; Haj-Zaroubi, M.; Awwad, S.; Landaub, S.Y.; Glazer, I. Toxicity of phenolic compounds to entomopathogenic nematodes: A case study with Heterorhabditis bacteriophora exposed to lentisk (Pistacia lentiscus) extracts and their chemical components. J. Invertebr. Pathol. 2019, 160, 43–53. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Du, G.C.; Li, Y.X.; Liang, C.Y.; Wang, C.; Zhang, Y.N.; Li, R.G. Nematotoxic coumarins from Angelica pubescens Maxim. f. biserrat a Shan et Yuan roots and their physiological effects on Bursaphelenchus xylophilus. J. Nematol. 2018, 50, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Takaishi, K.; Izumi, M.; Baba, N.; Kawazu, K.; Nakajima, S. Synthesis and biological evaluation of alkoxycoumarins as novel nematicidal constituents. Bioorg. Med. Chem. 2008, 18, 5614–5617. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, G.H.; Li, L.; Zheng, L.J.; Huang, R.; Zhang, K.Q. Nematicidal coumarins from Heracleum candicans Wall. Nat. Prod. Res. 2008, 22, 666–671. [Google Scholar] [CrossRef]
- Caboni, P.; Saba, M.; Oplos, C.; Aissani, N.; Maxia, A.; Menkissoglu-Spiroudi, U.; Casu, L.; Ntalli, N. Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest. Manag. Sci. 2015, 71, 1099–1105. [Google Scholar] [CrossRef]
- Koyuncu, E.A.; Yasar, A.; Arslan, F.; Sari, N. Synthesis of novel Schiff base derivatives of tacrine and investigation of their acetylcholinesterase inhibition potency. Maced. J. Chem. Chem. Eng. 2019, 38, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Riazimontazer, E.; Sadeghpour, H.; Nadri, H.; Sakhteman, A.; Tüylü Küçükkılınç, T.; Miri, R.; Edraki, N. Design, synthesis and biological activity of novel tacrine-isatin Schiff base hybrid derivatives. Bioorg. Chem. 2019, 89, 103006. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ullah, H.; Taha, M.; Wadood, A.; Javed, M.T.; Rehman, W.; Nawaz, M.; Ashraf, M.; Ali, M.; Sajid, M.; et al. Synthesis and in vitro acetylcholinesterase and butyrylcholinesterase inhibitory potential of hydrazide based Schiff bases. Bioorg. Chem. 2016, 68, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Kim, E.; Lee, S.H.; Park, I.K. Inhibition of acetylcholinesterases of the pinewood nematode, Bursaphelenchus xylophilus, by phytochemicals from plant essential oils. Pestic. Biochem. Physiol. 2013, 105, 50–56. [Google Scholar] [CrossRef]
- Sousa, B.L.; de Leite, J.P.V.; Mendes, T.A.O.; Varejão, E.V.V.; Chaves, A.C.S.; Silva, J.G.; da Agrizzi, A.P.; Ferreira, P.G.; Pilau, E.J.; Silva, E.; et al. Inhibition of acetylcholinesterase by coumarin-linked amino acids synthetized via triazole associated with molecule partition coefficient. J. Braz. Chem. Soc. 2021, 32, 652–664. [Google Scholar] [CrossRef]
- Bousada, G.M.; de Sousa, B.L.; Furlani, G.; Agrizzi, A.P.; Ferreira, P.G.; Leite, J.P.V.; Mendes, T.A.O.; Varejão, E.V.V.; Pilau, E.J.; Dos Santos, M.H. Tyrosol 1,2,3-triazole analogues as new acetylcholinesterase (AChE) inhibitors. Comput. Biol. Chem. 2020, 88, 107359. [Google Scholar] [CrossRef]
- Tehrani, M.B.; Rezaei, Z.; Asadi, M.; Behnammanesh, H.; Nadri, H.; Afsharirad, F.; Moradi, A.; Larijani, B.; Mohammadi-Khanaposhtani, M.; Mahdavi, M. Design, synthesis, and cholinesterase inhibition assay of coumarin-3-carboxamide-N-morpholine hybrids as new anti-alzheimer agents. Chem. Biodivers 2019, 16, e1900144. [Google Scholar] [CrossRef]
- Baruah, P.; Basumatary, G.; Yesylevskyy, S.O.; Aguan, K.; Bez, G.; Mitra, S. Novel coumarin derivatives as potent acetylcholinesterase inhibitors: Insight into efficacy, mode and site of inhibition. J. Biomol. Struct. Dyn. 2019, 37, 1750–1765. [Google Scholar] [CrossRef]
- Hao, G.; Dong, Q.; Yang, G.A. Comparative study on the constitutive properties of marketed pesticides. Mol. Inform. 2011, 30, 614–622. [Google Scholar] [CrossRef]
- Clarke, E.D.; Delaney, J.S. Physical and molecular properties of agrochemicals: An analysis of screen inputs, hits, leads, and products. Chimia 2003, 57, 731–734. [Google Scholar] [CrossRef]
- Bulgheroni, A.; Kinsner-Ovaskainen, A.; Hoffmann, S.; Hartung, T.; Prieto, P. Estimation of acute oral toxicity using the No Observed Adverse Effect Level (NOAEL) from the 28 day repeated dose toxicity studies in rats. Regul. Toxicol. Pharmacol. 2009, 53, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Costanza, J.; Lynch, D.G.; Boethling, R.; Arnot, J.A. Use of the bioaccumulation factor to screen chemicals for bioaccumulation potential. Environ. Toxico Chem. 2012, 31, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Siber, T.; Bušić, V.; Zobundžija, D.; Roca, S.; Vikić-Topić, D.; Vrandečić, K.; Gašo-Sokač, D. An improved method for the quaternization of nicotinamide and antifungal activities of its derivatives. Molecules 2019, 24, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bušić, V.; Vrandečić, K.; Siber, T.; Roca, S.; Vikić-Topić, D.; Gašo-Sokač, D. A rapid microwave induced synthesis of isonicotinamide derivatives and their antifungal activity. Croat. Chem. Acta 2019, 92, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Martin, T.M. User’s Guide for T.E.S.T. (Version 5.1) (Toxicity Estimation Software Tool): A Program to Estimate Toxicity from Molecular Structure; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2020.
- Schultz, T.W.; Sparfkin, C.L.; Aptula, A.O. Reactivity-based toxicity modelling of five-membered heterocyclic compounds: Application to Tetrahymena pyriformis. SAR QSAR Environ. Res. 2010, 21, 681–691. [Google Scholar] [CrossRef]
- ECHA-11-R-004.2-EN. The Use of Alternatives to Testing on Animals for the REACH Regulation; European Chemicals Agency: Helsinki, Finland, 2011. [Google Scholar]
- Hansen, K.; Mika, S.; Schroeter, T.; Sutter, A.; ter Laak, A.; Steger-Hartmann, T.; Heinrich, N.; Müller, K.R. Benchmark Data set for in silico prediction of ames mutagenicity. J. Chem. Model. 2009, 49, 2077–2081. [Google Scholar] [CrossRef]
- Arnot, J.A.; Gobas, F.A.P.C. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Hocquet, A.; Langgård, M. An Evaluation of the MM+ Force Field. J. Mol. Model. 1998, 4, 94–112. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colletier, J.P.; Fournier, D.; Greenblatt, H.M.; Stojan, J.; Sussman, J.L.; Zaccai, G.; Silman, I.; Weik, M. Structural insights into substrate traffic and inhibition in acetylcholinesterase. EMBO J. 2006, 25, 2743. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]



 |  | ||
| Numb. | R1/R2 | Numb. | R |
| 1 | R1 = NO2; R2 = Cl | 6 |  |
| 2 | R1 = Cl; R2 = CH3 |  | |
| 3 | R1 = Br; R2 = CH3 | Numb. | R |
| 4 | R1 = OCH3; R2 = CH3 | 8 | −N(CH3)2 |
| 5 | R1 = I; R2 = Cl | 9 | −OCH3 |
| 7 | R1 = I; R2 = CH3 | ||
| Antifungal Activity a | Antibacterial Activity b | Nematicidal Activity c | Inhibition of AChE/% ** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mol. No. | Macrophomina phaseolina | Sclerotinia sclerotiorum | Fusarium oxysporum f. sp. lycopersici | Fusarium culmorum | Bacillus mycoides | Bradyrhizobium japonicum | Heterorhabditis bacteriophora | Steinernema feltiae | |
| 1 | 67.47 ± 0.49 | 26.64 ± 3.44 | 20.63 ± 4.60 | 27.73 ± 8.20 | >512 | >512 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 |
| 2 | 67.47 ± 0.37 | 29.37 ± 6.06 | 24.27 ± 1.58 | 22.53 ± 9.12 | >512 | >512 | 8.75 ± 2.50 | 20.00 ± 0.00 | 0.00 |
| 3 | 71.51 ± 0.37 | 9.56 ± 5.23 | 23.06 ± 2.43 | 37.26 ± 6.01 | >512 | >512 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 |
| 4 | 70.36 ± 0.64 | 55.33 ± 9.56 | 26.70 ± 2.81 | 28.60 ± 4.90 | >512 | >512 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 |
| 5 | 68.05 ± 0.56 | 30.74 ± 11.01 | 30.34 ± 2.43 | 34.66 ± 1.74 | >512 | >512 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 |
| 6 | 67.47 ± 0.52 | 51.23 ± 9.03 | 25.49 ± 6.11 | 9.53 ± 0.71 | >512 | >512 | 0.00 ± 0.00 | 2.50 ± 0.50 | 0.00 |
| 7 | 69.20 ± 0.19 | 38.93 ± 6.06 | 21.84 ± 2.80 | 19.06 ± 1.99 | >512 | >512 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 |
| 8 | 65.17 ± 0.19 | 20.49 ± 6.87 | 9.71 ± 2.76 | 22.53 ± 7.93 | >512 | >512 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.59 |
| 9 | 66.32 ± 0.27 | 56.69 ± 7.52 | 16.99 ± 4.86 | 3.47 ± 0.19 | >512 | >512 | 46.25 ± 11.09 | 40.00 ± 7.07 | 31.45 |
| control | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | donepezil 99.89 |
| PC | Eigenvalue | % of Variance |
|---|---|---|
| 1 | 2.04 | 50.96 |
| 2 | 1.45 | 36.24 |
| 3 | 0.32 | 7.98 |
| 4 | 0.19 | 4.81 |
| Fungi | Coefficients of PC1 | Coefficients of PC 2 |
|---|---|---|
| Macrophomina phaseolina | 0.61 | 0.25 |
| Sclerotinia sclerotiorum | −0.29 | 0.70 |
| Fusarium oxysporum f. sp. lycopersici | 0.49 | 0.54 |
| Fusarium culmorum | 0.55 | −0.39 |
| Demethylase | Chitinase | Transferase | Endoglucanase I | Proteinase K | Pectinase | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp. (Pose) | Total E | Comp. (Pose) | Total E | Comp. (Pose) | Total E | Comp. (Pose) | Total E | Comp. (Pose) | Total E | Comp. (Pose) | Total E |
| 4 (0) | −131.26 | 2 (1) | −145.90 | 1 (0) | −131.17 | 3 (2) | −160.23 | 1 (1) | −148.87 | 3 (2) | −115.17 |
| 2 (0) | −128.04 | 5 (0) | −140.30 | 2 (1) | −111.41 | 1 (1) | −151.78 | 2 (0) | −135.07 | 2 (2) | −112.13 |
| 1 (0) | −124.73 | 7 (2) | −136.40 | 3 (2) | −105.96 | 7 (1) | −146.66 | 3 (1) | −124.76 | 8 (1) | −105.99 |
| 3 (1) | −120.73 | 4 (1) | −136.20 | 4 (1) | −118.54 | 4 (0) | −145.73 | 4 (0) | −134.79 | 6 (0) | −105.82 |
| 5 (1) | −118.59 | 1 (2) | −135.40 | 5 (0) | −125.71 | 2 (1) | −143.23 | 5 (0) | −135.03 | 5 (2) | −105.35 |
| 7 (2) | −118.57 | 3 (2) | −130.70 | 6 (1) | −103.44 | 5 (0) | −134.50 | 6 (1) | −117.53 | 9 (0) | −103.88 |
| 6 (0) | −113.25 | 8 (1) | −126.10 | 7 (0) | −113.52 | 8 (1) | −125.94 | 7 (0) | −120.70 | 7 (0) | −102.96 |
| 8 (2) | −98.68 | 9 (0) | −122.00 | 8 (1) | −106.26 | 6 (2) | −122.20 | 8 (2) | −110.73 | 1 (0) | −100.86 |
| 9 (1) | −97.66 | 6 (0) | −117.60 | 9 (2) | −98.78 | 9 (2) | −121.83 | 9 (2) | −113.81 | 4 (0) | −96.05 |
| Residue | Energy (kcal/mol) | Residue | Energy (kcal/mol) |
|---|---|---|---|
| H Bond | π-π Interactions | ||
| S-TRP-51 | −1.00 | S-TRP-51 | −16.88 |
| S-TYR-171 | −4.45 | S-HIS-213 | −7.12 |
| Van der Waals interactions | Alkyl and π-alkyl interactions | ||
| S-ARG-106 | −4.14 | M-ALA-211 | −0.27 |
| S-TYR-145 | −3.99 | M-CYS-172 | −5.45 |
| S-TYR-171 | −4.45 | π-cation interactions | |
| S-ASP-173 | −5.18 | S-ARG-106 | −10.23 |
| S-ASP-199 | −1.21 | π–σ interactions | |
| S-HIS-209 | −3.50 | M-TYR-177 | −7.26 |
| S-HIS-213 | −5.26 | S-TYR-177 | −11.84 |
| Compound | MW | MLOGP | HBA | HBD | RB | AB |
|---|---|---|---|---|---|---|
| 1 | 559 | 4.38 | 11 | 1 | 5 | 18 |
| 2 | 528 | 4.70 | 8 | 1 | 5 | 18 |
| 3 | 573 | 4.81 | 8 | 1 | 5 | 18 |
| 4 | 524 | 3.93 | 9 | 1 | 5 | 18 |
| 5 | 640 | 4.92 | 8 | 1 | 5 | 18 |
| 6 | 438 | 3.61 | 8 | 1 | 4 | 12 |
| 7 | 620 | 4.92 | 8 | 1 | 5 | 18 |
| 8 | 379 | 3.19 | 7 | 1 | 3 | 12 |
| 9 | 366 | 2.94 | 7 | 1 | 3 | 12 |
| Compound | Oral rat LD50 (mg/kg bw) a | Tetrahymena pyriformis IGC50 48-h (mg/L) b | Fathead Minnow LC50 96-h (mg/L) c | Mutagenicity Value (Result) d | Bioaccumulation (logBAF/L kg−1) e |
|---|---|---|---|---|---|
| 1 | 1050.80 | 7.67 | 8.63 × 10−4 | 0.74 (pos) | 1.04 |
| 2 | 1214.11 | 7.25 | 8.15 × 10−4 | 0.75 (pos) | 1.04 |
| 3 | 344.53 | 7.86 | 8.83 × 10−4 | 0.71 (pos) | 1.07 |
| 4 | 1183.17 | 7.19 | 8.08 × 10−4 | 0.74 (pos) | 0.66 |
| 5 | 2206.90 | 8.78 | 9.87 × 10−4 | 0.48 (neg) | 1.07 |
| 6 | 847.98 | 14.53 | 0.50 | 0.55 (pos) | 2.35 |
| 7 | 1164.29 | 8.50 | 9.56 × 10−4 | 0.43 (neg) | 1.07 |
| 8 | 643.35 | 5.20 | 0.22 | 0.70 (pos) | 1.27 |
| 9 | 623.67 | 5.02 | 0.30 | 0.53 (pos) | 1.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastija, V.; Vrandečić, K.; Ćosić, J.; Šarić, G.K.; Majić, I.; Agić, D.; Šubarić, D.; Karnaš, M.; Bešlo, D.; Komar, M.; et al. Effects of Coumarinyl Schiff Bases against Phytopathogenic Fungi, the Soil-Beneficial Bacteria and Entomopathogenic Nematodes: Deeper Insight into the Mechanism of Action. Molecules 2022, 27, 2196. https://doi.org/10.3390/molecules27072196
Rastija V, Vrandečić K, Ćosić J, Šarić GK, Majić I, Agić D, Šubarić D, Karnaš M, Bešlo D, Komar M, et al. Effects of Coumarinyl Schiff Bases against Phytopathogenic Fungi, the Soil-Beneficial Bacteria and Entomopathogenic Nematodes: Deeper Insight into the Mechanism of Action. Molecules. 2022; 27(7):2196. https://doi.org/10.3390/molecules27072196
Chicago/Turabian StyleRastija, Vesna, Karolina Vrandečić, Jasenka Ćosić, Gabriella Kanižai Šarić, Ivana Majić, Dejan Agić, Domagoj Šubarić, Maja Karnaš, Drago Bešlo, Mario Komar, and et al. 2022. "Effects of Coumarinyl Schiff Bases against Phytopathogenic Fungi, the Soil-Beneficial Bacteria and Entomopathogenic Nematodes: Deeper Insight into the Mechanism of Action" Molecules 27, no. 7: 2196. https://doi.org/10.3390/molecules27072196