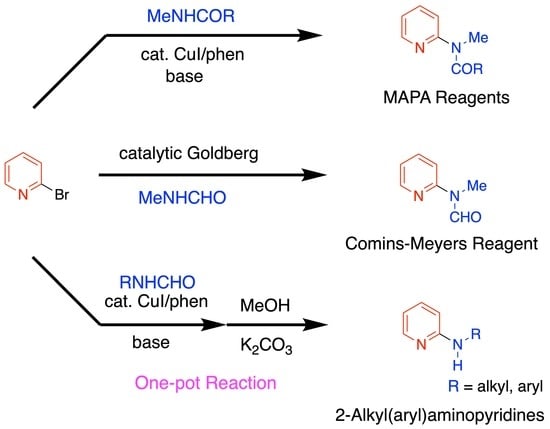
Synthesis of MAPA Reagents and 2-Alkyl(aryl)aminopyridines from 2-Bromopyridine Using the Goldberg Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of MAPA Reagents 1 and 2
2.2. One-Pot Synthesis of 2-Alkyl(aryl)aminopyridines from 2-Bromopyridine and Secondary Formamides
2.3. Use of DMEDA as an Alternative Ligand for the above Goldberg Reactions
3. Materials and Methods
3.1. General Information
3.2. Preparation of MAPA Compounds
3.2.1. N-Methyl-N-(pyridin-2-yl)acetamide (2a)
3.2.2. N-Methyl-N-(pyridin-2-yl)benzamide (2b)
3.2.3. N-Methyl-N-(pyridin-2-yl)formamide (1)
3.3. Preparation of 2-Alkyl(aryl)aminopyridines
3.3.1. N-Butylpyridin-2-amine (3b)
3.3.2. N-Benzylpyridin-2-amine (3c)
3.3.3. N-(p-Tolyl)pyridine-2-amine (3e)
3.3.4. N-(tert-Butyl)pyridine-2-amine (3d)
3.3.5. N-Methylpyridin-2-amine (3a)
3.4. Preparation of MAPA Compounds from 2-Bromopyridine Using DMEDA as Ligand
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Comins, D.; Meyers, A.I. A Facile and Efficient Formylation of Grignard Reagents. Synthesis 1978, 1978, 403–404. [Google Scholar] [CrossRef]
- Meyers, A.I.; Comins, D.L. N-Methylamino Pyridyl Amides (MAPA) II. An Efficient Acylating Agent for Various Nucleophiles and Sequential Addition to Unsymmetrical-Alcohols. Tetrahedron Lett. 1978, 19, 5179–5182. [Google Scholar] [CrossRef]
- Comins, D.L.; Dernell, W. A One Pot Synthesis Unsymmetric Secondary Alcohols from Two Grignard Reagents. Tetrahedron Lett. 1981, 22, 1085–1088. [Google Scholar] [CrossRef]
- Comins, D.L.; Joseph, S.P. N-Methyl-N-(2-pyridyl)formamide. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Chichester, UK, 2001; p. rm239m. [Google Scholar] [CrossRef]
- Monticelli, S.; Parisi, G.; Rui, M.; de la Vega-Hernández, K.; Murgia, I.; Senatore, R.; Holzer, W.; Urban, E.; Langer, T.; Langer, T.; et al. The Use of the Comins-Meyers Amide in Synthetic Chemistry: An Overview. Nat. Prod. Commun. 2016, 11, 1729–1732. [Google Scholar] [CrossRef] [Green Version]
- Manley, P.J.; Bilodeau, M.T. A Mild Method for the Formation and in Situ Reaction of Imidoyl Chlorides: Conversion of Pyridine-1-Oxides to 2-Aminopyridine Amides. Org. Lett. 2002, 4, 3127–3129. [Google Scholar] [CrossRef]
- Baqi, Y. Recent Advances in Microwave-Assisted Copper-Catalyzed Cross-Coupling Reactions. Catalysts 2020, 11, 46. [Google Scholar] [CrossRef]
- Thomas, A.M.; Sujatha, A.; Anilkumar, G. Goldberg Reaction: Development, Mechanistic Insights and Applications. Mini-Rev. Org. Chem. 2015, 12, 3–23. [Google Scholar] [CrossRef]
- Casitas, A.; Ribas, X. Insights into the Mechanism of Modern Ullmann-Goldberg Coupling Reactions. In Copper-Mediated Cross-Coupling Reactions; Evano, G., Blanchard, N., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 253–279. [Google Scholar] [CrossRef]
- Evano, G.; Theunissen, C.; Pradal, A. Impact of Copper-Catalyzed Cross-Coupling Reactions in Natural Product Synthesis: The Emergence of New Retrosynthetic Paradigms. Nat. Prod. Rep. 2013, 30, 1467. [Google Scholar] [CrossRef]
- Jiang, L. Copper/N,N-Dimethylglycine Catalyzed Goldberg Reactions Between Aryl Bromides and Amides, Aryl Iodides and Secondary Acyclic Amides. Molecules 2014, 19, 13448–13460. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Bao, W. A β-Keto Ester as a Novel, Efficient, and Versatile Ligand for Copper(I)-Catalyzed C−N, C−O, and C−S Coupling Reactions. J. Org. Chem. 2007, 72, 3863–3867. [Google Scholar] [CrossRef]
- Nicolas, L.; Angibaud, P.; Stansfield, I.; Meerpoel, L.; Reymond, S.; Cossy, J. Copper-Catalysed Amidation of 2-Chloro-Pyridines. RSC Adv. 2013, 3, 18787. [Google Scholar] [CrossRef]
- Kiyomori, A.; Marcoux, J.-F.; Buchwald, S.L. An Efficient Copper-Catalyzed Coupling of Aryl Halides with Imidazoles. Tetrahedron Lett. 1999, 40, 2657–2660. [Google Scholar] [CrossRef]
- Klapars, A.; Antilla, J.C.; Huang, X.; Buchwald, S.L. A General and Efficient Copper Catalyst for the Amidation of Aryl Halides and the N-Arylation of Nitrogen Heterocycles. J. Am. Chem. Soc. 2001, 123, 7727–7729. [Google Scholar] [CrossRef] [PubMed]
- Klapars, A.; Huang, X.; Buchwald, S.L. A General and Efficient Copper Catalyst for the Amidation of Aryl Halides. J. Am. Chem. Soc. 2002, 124, 7421–7428. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Tajbakhsh, M.; Alikarami, M. Copper-Catalyzed N-Arylation of Diazoles with Aryl Bromides Using KF/Al2O3: An Improved Protocol. Tetrahedron Lett. 2006, 47, 5203–5205. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Tajbakhsh, M.; Mohadjerani, M.; Mehdinejad, H. Copper-Catalyzed Amidation of Aryl Iodides Using KF/Al2O3: An Improved Protocol. Synlett 2004, 9, 1517–1520. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Aghili, N.; Tajbakhsh, M. SBA-15 Immobilized Phenanthroline–Copper(I) Complex as a Recyclable Efficient Catalyst for N-Arylation of Amides and N–H Heterocycles with Aryl Halides. Catal. Lett. 2016, 146, 193–203. [Google Scholar] [CrossRef]
- Rovira, M.; Soler, M.; Güell, I.; Wang, M.-Z.; Gómez, L.; Ribas, X. Orthogonal Discrimination among Functional Groups in Ullmann-Type C–O and C–N Couplings. J. Org. Chem. 2016, 81, 7315–7325. [Google Scholar] [CrossRef]
- Neetha, M.; Saranya, S.; Ann Harry, N.; Anilkumar, G. Recent Advances and Perspectives in the Copper-Catalysed Amination of Aryl and Heteroaryl Halides. ChemistrySelect 2020, 5, 736–753. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, R.; Xue, W.; Liu, X.; Zhao, Y.; Wang, K.-H.; Huang, D.; Huo, C.; Hu, Y. Visible-Light-Promoted Acyl Radical Cascade Reaction for Accessing Acylated Isoquinoline-1,3(2 H,4 H)-Dione Derivatives. Org. Biomol. Chem. 2020, 18, 1940–1948. [Google Scholar] [CrossRef]
- Waibel, K.A.; Nickisch, R.; Möhl, N.; Seim, R.; Meier, M.A.R. A More Sustainable and Highly Practicable Synthesis of Aliphatic Isocyanides. Green Chem. 2020, 22, 933–941. [Google Scholar] [CrossRef] [Green Version]
- Kamer, P.C.J.; Nolte, R.J.M.; Drenth, W. Screw Sense Selective Polymerization of Achiral Isocyanides Catalyzed by Optically Active Nickel(II) Complexes. J. Am. Chem. Soc. 1988, 110, 6818–6825. [Google Scholar] [CrossRef] [Green Version]
- Moffat, J.; Newton, M.V.; Papenmeier, G.J. Formylation of t-Butylamine and t-Octylamine. J. Org. Chem. 1962, 27, 4058. [Google Scholar] [CrossRef]
- Bernstein, J.; Stearns, B.; Dexter, M.; Lott, W.A.I. Derivatives of Aminopyridines. J. Am. Chem. Soc. 1947, 69, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Cano, R.; Ramón, D.J.; Yus, M. Impregnated Ruthenium on Magnetite as a Recyclable Catalyst for the N-Alkylation of Amines, Sulfonamides, Sulfinamides, and Nitroarenes Using Alcohols as Electrophiles by a Hydrogen Autotransfer Process. J. Org. Chem. 2011, 76, 5547–5557. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.N.; Rasheed, S.; Aravinda, S.; Vishwakarma, R.A.; Das, P. Base and Ligand Free Copper-Catalyzed N-Arylation of 2-Amino-N-Heterocycles with Boronic Acids in Air. RSC Adv. 2013, 3, 11472. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Eynde, J.-J.V. The Chemistry of N-Substituted Benzotriazoles. Part 20. Mono-N-t-Butylation of Aromatic and Heteroaromatic Amines. J. Chem. Soc. Perkin Trans. 1989, 1, 639–642. [Google Scholar] [CrossRef]
- Bergstrom, F.W.; Sturz, H.G.; Tracy, H.W. The use of the Fused Eutectic of Sodium Amide and Potassium Amide in Organic Syntheses. J. Org. Chem. 1946, 11, 239–246. [Google Scholar] [CrossRef]
- Wang, D.; Kuang, D.; Zhang, F.; Yang, C.; Zhu, X. Room-Temperature Copper-Catalyzed Arylation of Dimethylamine and Methylamine in Neat Water. Adv. Synth. Catal. 2015, 357, 714–718. [Google Scholar] [CrossRef]
- Okano, K.; Tokuyama, H.; Fukuyama, T. Synthesis of Secondary Arylamines through Copper-Mediated Intermolecular Aryl Amination. Org. Lett. 2003, 5, 4987–4990. [Google Scholar] [CrossRef]
- Zeng, J.; Tan, Y.J.; Leow, M.L.; Liu, X.-W. Copper(II)/Iron(III) Co-Catalyzed Intermolecular Diamination of Alkynes: Facile Synthesis of Imidazopyridines. Org. Lett. 2012, 14, 4386–4389. [Google Scholar] [CrossRef] [PubMed]

 | ||||||
|---|---|---|---|---|---|---|
| Entry a | Amide (R) | CuI/phen (mol%) b | Solvent | Base (Equiv.) | Time c (h) | Yield d (%) |
| 1 | H | 2 | t-AmOH | K3PO4 (2) | 8 | 1, 65 (28) e |
| 2 | H | 2 | PhMe/ t-AmOH (9:1) | K2CO3 (1.3) | 8 | 1, 93 |
| K3PO4 (0.1) | ||||||
| 3 | H | 1 | PhMe | K2CO3 (1.3) | 8 | 1, 92 |
| K3PO4 (0.05) | ||||||
| 4 | H | 0.5 | PhMe | K2CO3 (1.3) | 8 | 1, 88 |
| 5 | H | 2 | PhMe | K2CO3 (2) | 12 | 1, 85 f |
| K3PO4 (0.1) | ||||||
| 6 | Me | 2 | t-AmOH | K3PO4 (2) | 24 | 2a, 75 (10) e |
| 7 | Me | 2 | PhMe/ t-AmOH (9:1) | K3PO4 (2) | 24 | 2a, 84 |
| 8 | Me | 2 | 5% t-AmOH/ PhMe | K3PO4 (2) | 36 | 2a, 92 (88) g |
| 9 | Ph | 2 | t-AmOH | K3PO4 (2) | 36 | 2b, 68 (11)e |
| 10 | Ph | 2 | PhMe/ t-AmOH (9:1) | K3PO4 (2) | 36 | 2b, 85 |
| 11 | Ph | 2 | PhMe | K3PO4 (2) | 36 | 2b, 88 (80) g |
 | ||||||
|---|---|---|---|---|---|---|
| Entry a | Formamide R | CuI/phen (mol%) b | Solvent, Base; 1st Step | Time c (h) 1st Step | Time c (h) 2nd Step | Yield d (%) |
| 1 | Me | 2 | PhMe | 12 | 4 | 3a, 89 e |
| K3PO4 (2) | ||||||
| 2 | Bu | 2 | PhMe | 14 | 6 | 3b, 90 f |
| K3PO4 (2) | ||||||
| 3 | Bn | 2 | PhMe | 18 | 4 | 3c, 89 g |
| K3PO4 (2) | ||||||
| 4 | t-Bu | 3 | 5% t-AmOH/ PhMe | 48 h | 6 i | 3d, 85 f |
| K3PO4 (2) | ||||||
| 5 | p-tol | 1 | PhMe | 10 | 3 | 3e, 88 f |
| K2CO3 (1.5) | ||||||
| K3PO4 (0.5) | ||||||
 | ||||||
|---|---|---|---|---|---|---|
| Entry a | Amide R1, R2 | CuI/DMEDA mol%, Ratio | Solvent | Base (Equiv.) | Time b (h) | Yield c (%) |
| 1 | Me, H | 2/4 | PhMe | K2CO3 (1.3) | 8 | 1, 92 |
| K3PO4 (0.1) | ||||||
| 2 | Me, H | 2/2 | PhMe | K2CO3 (1.3) | 8 | 1, 91 |
| K3PO4 (0.1) | ||||||
| 3 | Me, H | 2 (no ligand) | PhMe | K2CO3 (2) | 8 | 1, 16 |
| K3PO4 (0.1) | ||||||
| 4 | Me, Me | 2/4 | PhMe | K3PO4 (2) | 24 | 2a, 8 |
| 5 | Me, Me | 2/2 | 5% t-AmOH/ PhMe | K3PO4 (2) | 24 | 2a, 10 |
| 6 | Me, Ph | 5/10 | PhMe | K3PO4 (2) | 36 | 2b, 81 |
| 7 | Bn, H | 2/4 | PhMe | K2CO3 (2) | 18 | 2c, 80 |
| 8 | Bn, H | 2/4 | PhMe | K3PO4 (2) | 18 | 2c, 84 |
| 9 | t-Bu, H | 5/10 | 5% t-AmOH/ PhMe | K3PO4 (2) | 24 | 2d, 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comins, D.L. Synthesis of MAPA Reagents and 2-Alkyl(aryl)aminopyridines from 2-Bromopyridine Using the Goldberg Reaction. Molecules 2022, 27, 1833. https://doi.org/10.3390/molecules27061833
Comins DL. Synthesis of MAPA Reagents and 2-Alkyl(aryl)aminopyridines from 2-Bromopyridine Using the Goldberg Reaction. Molecules. 2022; 27(6):1833. https://doi.org/10.3390/molecules27061833
Chicago/Turabian StyleComins, Daniel L. 2022. "Synthesis of MAPA Reagents and 2-Alkyl(aryl)aminopyridines from 2-Bromopyridine Using the Goldberg Reaction" Molecules 27, no. 6: 1833. https://doi.org/10.3390/molecules27061833