Use of Gas Chromatography-Mass Spectrometry Techniques (GC-MS, GC-MS/MS and GC-QTOF) for the Characterization of Photooxidation and Autoxidation Products of Lipids of Autotrophic Organisms in Environmental Samples
Abstract
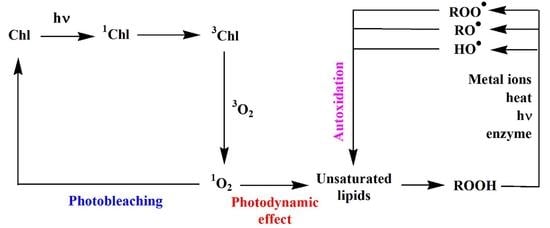
:1. Introduction
2. Abiotic Oxidation of Lipid Components of Autotrophic Organisms
2.1. Type II Photosensitized Oxidation
2.2. Free Radical Oxidation (Autoxidation)
3. Characterization of the Oxidation Products of Lipids
3.1. Chlorophyll Phytyl Side-Chain
3.2. ∆5-Sterols
3.3. Unsaturated Fatty Acids
3.4. Pentacyclic Triterpenes
3.4.1. Lupanes
3.4.2. Ursanes and Oleanes
3.5. Dehydroabietic Acid
3.6. Highly Branched Isoprenoid (HBI) Alkenes
3.7. Alkenones
3.8. Carotenoids
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shimakawa, G.; Matsuda, Y.; Nakajima, K.; Tamoi, M.; Shigeoka, S.; Miyake, C. Diverse strategies of O2 usage for preventing photo-oxidative damage under CO2 limitation during algal photosynthesis. Sci. Rep. 2017, 7, 41022. [Google Scholar] [CrossRef] [Green Version]
- Harwood, J.L.; Russell, N.J. Lipids in Plants and Microbes; Springer: Dordrecht, The Netherlands, 1984; pp. 7–32. [Google Scholar]
- Jònasdòttir, S.H. Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 2019, 17, 151. [Google Scholar] [CrossRef] [Green Version]
- Ben-Amotz, A.; Tornabene, T.G.; Thomas, W.H. Chemical profile of selected species of microalgae with emphasis on lipids. J. Phycol. 1985, 21, 72–81. [Google Scholar] [CrossRef]
- Volkman, J.K. Lipid markers for marine organic matter. Handb. Environ. Chem. 2006, 2, 27–70. [Google Scholar]
- Parrish, C.C. Lipids in marine ecosystems. Int. Sch. Res. Not. 2013, 2013, 604045. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Glendell, M.; Meersmans, J.; Kirkels, F.; Middelburg, J.J.; Peterse, F. Assessing branched tetraether lipids as tracers of soil organic carbon transport through the Carminowe Creek Catchment (Southwest England). Biogeosciences 2020, 17, 3183–3201. [Google Scholar] [CrossRef]
- Rontani, J.-F. Photo- and Free Radical-Mediated Oxidation of Lipid Components during the Senescence of Phototrophic Organisms. In Senescence; Nagata, T., Ed.; Intech: Rijeka, Croatia, 2012; pp. 3–31. [Google Scholar]
- Rontani, J.-F.; Belt, S.T. Photo- and autoxidation of unsaturated algal lipids in the marine environment: An overview of processes, their potential tracers, and limitations. Org. Geochem. 2020, 139, 103941. [Google Scholar] [CrossRef]
- Marchand, D.; Rontani, J.-F. Characterisation of photo-oxidation and autoxidation products of phytoplanktonic monounsaturated fatty acids in marine particulate matter and recent sediments. Org. Geochem. 2001, 32, 287–304. [Google Scholar] [CrossRef]
- Xia, W.; Budge, S.M. Techniques for the analysis of minor lipid oxidation products derived from triacylglycerols: Epoxides, alcohols, and ketones. Compr. Rev. Food Sci. Food Saf. 2017, 16, 735–758. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring oxidative stability and changes in key volatile compounds in edible oils during ambient storage through HS-SPME/GC–MS. Int. J. Food Prop. 2017, 20, S2926–S2938. [Google Scholar] [CrossRef] [Green Version]
- Barden, A.; Mori, T.A. GC-MS analysis of lipid oxidation products in blood, urine, and tissue samples. In Clinical Metabolomics; Humana Press: New York, NY, USA, 2018; pp. 283–292. [Google Scholar]
- Koek, M.M.; Jellema, R.H.; van der Greef, J.; Tas, A.C.; Hankemeier, T. Quantitative metabolomics based on gas chromatography mass spectrometry: Status and perspectives. Metabolomics 2011, 7, 307–328. [Google Scholar] [CrossRef] [Green Version]
- Frankel, E.N. Lipid Oxidation; Woodhead Publishing: Cambridge, UK, 2014; pp. 129–161. [Google Scholar]
- Schick, D.; Link, K.; Schwack, W.; Granvogl, M.; Oellig, C. Analysis of mono-, di-, triacylglycerols, and fatty acids in food emulsifiers by high-performance liquid chromatography–mass spectrometry. Eur. Food Res. Technol. 2021, 247, 1023–1034. [Google Scholar] [CrossRef]
- Han, E.C.; Lee, Y.S.; Liao, W.S.; Liu, Y.C.; Liao, H.Y.; Jeng, L.B. Direct tissue analysis by MALDI-TOF mass spectrometry in human hepatocellular carcinoma. Clin. Chim. Acta 2011, 412, 230–239. [Google Scholar] [CrossRef]
- Leopold, J.; Popkova, Y.; Engel, K.M.; Schiller, J. Recent developments of useful MALDI matrices for the mass spectrometric characterization of lipids. Biomolecules 2018, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Paglia, G.; Kliman, M.; Claude, E.; Geromanos, S.; Astarita, G. Applications of ion-mobility mass spectrometry for lipid analysis. Anal. Bioanal. Chem. 2015, 407, 4995–5007. [Google Scholar] [CrossRef]
- Leaptrot, K.L.; May, J.C.; Dodds, J.N.; McLean, J.A. Ion mobility conformational lipid atlas for high confidence lipidomics. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Merkx, D.W.; Hong, G.S.; Ermacora, A.; Van Duynhoven, J.P. Rapid quantitative profiling of lipid oxidation products in a food emulsion by 1H NMR. Anal. Chem. 2018, 90, 4863–4870. [Google Scholar] [CrossRef]
- He, P.; Aga, D.S. Comparison of GC-MS/MS and LC-MS/MS for the analysis of hormones and pesticides in surface waters: Advantages and pitfalls. Anal. Methods 2019, 11, 1436–1448. [Google Scholar] [CrossRef]
- Pierce, A.E. Silylation of Organic Compounds; Pierce Chemical Company: Rockford, IL, USA, 1982; pp. 72–215. [Google Scholar]
- Evershed, R. Biomolecular archaeology and lipids. World Archaeol. 1993, 25, 74–93. [Google Scholar] [CrossRef]
- Goad, L.J.; Akihisa, T. Mass Spectrometry of Sterols. In Analysis of Sterols; Goad, L.J., Akihisa, T., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 152–196. [Google Scholar]
- Harvey, D.J.; Vouros, P. Mass spectrometric fragmentation of trimethylsilyl and related alkylsilyl derivatives. Mass Spectrom. Rev. 2020, 39, 105–211. [Google Scholar] [CrossRef]
- Foote, C.S. Photosensitized Oxidation and Singlet Oxygen: Consequences in Biological Systems. In Free Radicals in Biology; Pryor, W.A., Ed.; Academic Press: New York, NY, USA, 1976; pp. 85–133. [Google Scholar]
- Knox, J.P.; Dodge, A.D. Singlet oxygen and plants. Phytochemistry 1985, 24, 889–896. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative damage, lipid peroxidation and antioxidant protection in chloroplasts. Chem. Phys. Lipids 1987, 44, 327–340. [Google Scholar] [CrossRef]
- Nelson, J.R. Rates and possible mechanism of light-dependent degradation of pigments in detritus derived from phytoplankton. J. Mar. Res. 1993, 51, 155–179. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Hendry, G.A.F. Free radical metabolism, pigment degradation and lipid peroxidation in leaves during senescence. Proc. R. Soc. Edinb. 1994, 102B, 459–471. [Google Scholar] [CrossRef]
- Glaeser, J.; Nuss, A.M.; Berghoff, B.A.; Klug, G. Singlet oxygen stress in microorganisms. Adv. Microb. Physiol. 2011, 58, 141–173. [Google Scholar]
- Hurst, J.R.; Wilson, S.L.; Schuster, G.B. The ene reaction of singlet oxygen: Kinetic and product evidence in support of a perepoxide intermediate. Tetrahedron 1985, 41, 2191–2197. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Overview of Reactive Oxygen Species. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Santi, N., Flors, C., Eds.; The Royal Society of Chemistry: London, UK, 2016; Volume 1, pp. 1–21. [Google Scholar]
- Schaich, K.M. Lipid Oxidation: Theoretical Aspects. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons: Chichester, UK, 2005; pp. 269–355. [Google Scholar]
- Fossey, J.; Lefort, D.; Sorba, J. Free Radicals in Organic Chemistry; John Wiley & Sons: Chichester, UK, 1995; pp. 191–200. [Google Scholar]
- Girotti, A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998, 39, 1529–1542. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Rabourdin, A.; Marchand, D.; Aubert, C. Photochemical oxidation and autoxidation of chlorophyll phytyl side chain in senescent phytoplanktonic cells: Potential sources of several acyclic isoprenoid compounds in the marine environment. Lipids 2003, 38, 241–254. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Cuny, P.; Grossi, V. Photodegradation of chlorophyll phytyl chain in senescent leaves of higher plants. Phytochemistry 1996, 42, 347–351. [Google Scholar] [CrossRef]
- Cuny, P.; Rontani, J.-F. On the widespread occurrence of 3-methylidene-7,11,15-trimethylhexadecan-1,2-diol in the marine environment: A specific isoprenoid marker of chlorophyll photodegradation. Mar. Chem. 1999, 65, 155–165. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Aubert, C. Characterization of isomeric allylic diols resulting from chlorophyll phytyl side-chain photo- and autoxidation by electron ionization gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Rontani, J.-F.; Galeron, M.-A. Autoxidation of chlorophyll phytyl side chain in senescent phototrophic organisms: A potential source of isophytol in the environment. Org. Geochem. 2016, 97, 35–40. [Google Scholar] [CrossRef]
- Kulig, M.J.; Smith, L.L. Sterol metabolism. XXV. Cholesterol oxidation by singlet molecular oxygen. J. Org. Chem. 1973, 38, 3639–3642. [Google Scholar] [CrossRef] [PubMed]
- Korytowski, W.; Bachowski, G.J.; Girotti, A.W. Photoperoxidation of cholesterol in homogeneous solution, isolated membranes, and cells: Comparison of the 5α- and 6β-hydroperoxides as indicators of singlet oxygen intermediacy. Photochem. Photobiol. 1992, 56, 1–8. [Google Scholar] [CrossRef]
- Christodoulou, S.; Marty, J.-C.; Miquel, J.-C.; Volkman, J.K.; Rontani, J.-F. Use of lipids and their degradation products as biomarkers for carbon cycling in the northwestern Mediterranean Sea. Mar. Chem. 2009, 113, 25–40. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Zabeti, N.; Wakeham, S.G. The fate of marine lipids: Biotic vs. abiotic degradation of particulate sterols and alkenones in the northwestern Mediterranean Sea. Mar. Chem. 2009, 113, 9–18. [Google Scholar] [CrossRef]
- Smith, L.L. Cholesterol autoxidation 1981–1986. Chem. Phys. Lipids 1981, 44, 87–125. [Google Scholar] [CrossRef]
- Morrisey, P.A.; Kiely, M. Oxysterols: Formation and biological function. Adv. Dairy Chem. 2006, 2, 641–674. [Google Scholar]
- Harvey, D.J.; Vouros, P. Influence of the 6-trimethylsilyl group on the fragmentation of the trimethylsilyl derivatives of some 6-hydroxy- and 3,6-dihydroxy-steroids and related compounds. Biomed. Mass Spectrom. 1979, 6, 135–143. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Charrière, B.; Sempéré, R.; Doxaran, D.; Vaultier, F.; Vonk, J.E.; Volkman, J.K. Degradation of sterols and terrigenous organic matter in waters of the Mackenzie Shelf, Canadian Arctic. Org. Geochem. 2014, 75, 61–73. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation; The Oily Press: Dundee, UK, 1998; pp. 23–41. [Google Scholar]
- Rontani, J.-F.; Cuny, P.; Grossi, V. Identification of a “pool” of lipid photoproducts in senescent phytoplanktonic cells. Org. Geochem. 1998, 29, 1215–1225. [Google Scholar] [CrossRef]
- Vigor, C.; Bertrand-Michel, J.; Pinot, E.; Oger, C.; Vercauteren, J.; Le Faouder, P.; Galano, J.M.; Lee, J.C.-Y.; Durand, T. Non-enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B 2014, 964, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Imbusch, R.; Mueller, M.J. Formation of isoprostane F2-like compounds (phytoprostanes F1) from α-linolenic acid in plants. Free Radic. Biol. Med. 2000, 28, 720–726. [Google Scholar] [CrossRef]
- Frankel, E.N.; Neff, W.E.; Bessler, T.R. Analysis of autoxidized fats by gas chromatography-mass spectrometry: V. Photosensitized oxidation. Lipids 1979, 14, 961–967. [Google Scholar] [CrossRef]
- Porter, N.A.; Caldwell, S.E.; Mills, K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids 1995, 30, 277–290. [Google Scholar] [CrossRef]
- Christodoulou, S.; Joux, F.; Marty, J.-C.; Sempéré, R.; Rontani, J.-F. Comparative study of UV and visible light induced degradation of lipids in non-axenic senescent cells of Emiliania huxleyi. Mar. Chem. 2010, 119, 139–152. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants–rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [Green Version]
- Galeron, M.-A.; Volkman, J.K.; Rontani, J.-F. Oxidation products of betulin: New tracers of abiotic degradation of higher plant material in the environment. Org. Geochem. 2016, 91, 31–42. [Google Scholar] [CrossRef]
- Rontani, J.-F. Lipid Oxidation Products: Useful Tools for Monitoring Photo- and Autoxidation in Phototrophs; Cambridge Scholar Publishing: Newcastle upon Tyne, UK, 2021; pp. 51–70. [Google Scholar]
- Galeron, M.-A.; Vaultier, F.; Rontani, J.-F. Oxidation products of α- and β-amyrins: Potential tracers of abiotic degradation of vascular-plant organic matter in aquatic environments. Environ. Chem. 2016, 13, 732–744. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Charrière, B.; Menniti, C.; Aubert, D.; Aubert, C. Electron ionization mass spectrometry fragmentation and multiple reaction monitoring quantification of autoxidation products of α-and β-amyrins in natural samples. Rapid Commun. Mass Spectrom. 2018, 32, 1599–1607. [Google Scholar] [CrossRef]
- Budzikiewicz, H.; Wilson, J.M.; Djerassi, C. Mass spectrometry in structural and stereochemical problems. XXXII.1 Pentacyclic triterpenes. J. Am. Chem. Soc. 1963, 85, 3688–3699. [Google Scholar] [CrossRef]
- Brassell, S.C.; Eglinton, G.; Maxwell, J.R. The geochemistry of terpenoids and steroids. Biochem. Soc. Trans. 1983, 1, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Simoneit, B.R.T.; Rember, W.C. Conifer and angiosperm biomarkers in clay sediments and fossil plants from the Miocene Clarkia Formation, Idaho, USA. Org. Geochem. 2005, 36, 907–922. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Aubert, C.; Belt, S.T. EIMS Fragmentation pathways and MRM quantification of 7α/β-hydroxy-dehydroabietic acid TMS derivatives. Rapid Commun. Mass Spectrom. 2018, 26, 1606–1616. [Google Scholar] [CrossRef]
- Belt, S.T.; Müller, J. The Arctic sea ice biomarker IP25: A review of current understanding, recommendations for future research and applications in palaeo sea ice reconstructions. Quat. Sci. Rev. 2013, 79, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Belt, S.T. Source-specific biomarkers as proxies for Arctic and Antarctic sea ice. Org. Geochem. 2018, 125, 277–298. [Google Scholar] [CrossRef] [Green Version]
- Schulte-Elte, K.H.; Muller, B.L.; Pamingle, H. Photooxygenation of 3, 3-dialkylsubstituted allyl alcohols. Occurrence of syn preference in the ene addition of 1O2 at E/Z-isomeric allyl alcohols. Helv. Chim. Acta 1979, 62, 816–829. [Google Scholar] [CrossRef]
- Belt, S.T.; Brown, T.A.; Smik, L.; Tatarek, A.; Wiktor, J.; Stowasser, G.; Husum, K. Identification of C25 highly branched isoprenoid (HBI) alkenes in diatoms of the genus Rhizosolenia in polar and sub-polar marine phytoplankton. Org. Geochem. 2017, 110, 65–72. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Belt, S.T.; Brown, T.A.; Vaultier, F.; Mundy, C.J. Sequential photo-and autoxidation of diatom lipids in Arctic sea ice. Org. Geochem. 2014, 77, 59–71. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Belt, S.T.; Brown, T.A.; Aubert, C. Electron ionization mass spectrometry fragmentation pathways of trimethylsilyl derivatives of isomeric allylic alcohols derived from HBI alkene oxidation. Rapid Commun. Mass Spectrom. 2014, 28, 1937–1947. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Smik, L.; Belt, S.T.; Vaultier, F.; Armbrecht, L.; Leventer, A.; Armand, L.K. Abiotic degradation of highly branched isoprenoid alkenes and other lipids in the water column off East Antarctica. Mar. Chem. 2019, 210, 34–47. [Google Scholar] [CrossRef]
- Volkman, J.K.; Eglinton, G.; Corner, E.D.; Forsberg, T.E.V. Long-chain alkenes and alkenones in the marine coccolithophorid Emiliania huxleyi. Phytochemistry 1980, 19, 2619–2622. [Google Scholar] [CrossRef]
- Volkman, J.K.; Barrett, S.M.; Blackburn, S.I.; Sikes, E.L. Alkenones in Gephyrocapsa oceanica: Implications for studies of paleoclimate. Geochim. Cosmochim. Acta 1995, 59, 513–520. [Google Scholar] [CrossRef]
- Marlowe, I.T.; Green, J.C.; Neal, A.C.; Brassell, S.C.; Eglinton, G.; Course, P.A. Long chain (n-C37–C39) alkenones in the Prymnesiophyceae. Distribution of alkenones and other lipids and their taxonomic significance. Br. Phycol. J. 1984, 19, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Prahl, F.G.; Mix, A.C.; Sparrow, M.A. Alkenone paleothermometry: Biological lessons from marine sediment records off western South America. Geochim. Cosmochim. Acta 2006, 70, 101–117. [Google Scholar] [CrossRef]
- Jaraula, C.M.; Brassell, S.C.; Morgan-Kiss, R.M.; Doran, P.T.; Kenig, F. Origin and tentative identification of tri- to penta-unsaturated ketones in sediments from Lake Fryxell, East Antarctica. Org. Geochem. 2010, 41, 386–397. [Google Scholar] [CrossRef]
- Prahl, F.G.; Wakeham, S.G. Calibration of unsaturation patterns in long-chain ketone compositions for palaeotemperature assessment. Nature 1987, 330, 367–369. [Google Scholar] [CrossRef]
- Prahl, F.G.; Muehlhausen, L.A.; Zahnle, D.L. Further evaluation of long-chain alkenones as indicators of paleoceanographic conditions. Geochim. Cosmochim. Acta 1988, 52, 2303–2310. [Google Scholar] [CrossRef]
- Brassell, S.C. Applications of biomarkers for delineating marine paleoclimatic fluctuations during the Pleistocene. In Organic Geochemistry; Topics in Geobiology; Engel, M.H., Macko, S.A., Eds.; Springer: Boston, MA, USA, 1993; Volume 23, pp. 699–738. [Google Scholar]
- Müller, P.J.; Kirst, G.; Ruhland, G.; Von Storch, I.; Rosell-Melé, A. Calibration of the alkenone paleotemperature index based on core-tops from the eastern South Atlantic and the global ocean (60° N–60° S). Geochim. Cosmochim. Acta 1998, 62, 1757–1772. [Google Scholar] [CrossRef]
- Rechka, J.A.; Maxwell, J.R. Characterisation of alkenone temperature indicators in sediments and organisms. Org. Geochem. 1988, 13, 727–734. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Cuny, P.; Grossi, V.; Beker, B. Stability of long-chain alkenones in senescing cells of Emiliania huxleyi: Effect of photochemical and aerobic microbial degradation on the alkenone unsaturation ratio . Org. Geochem. 1997, 26, 503–509. [Google Scholar] [CrossRef]
- Mouzdahir, A.; Grossi, V.; Bakkas, S.; Rontani, J.-F. Visible light-dependent degradation of long-chain alkenes in killed cells of Emiliania huxleyi and Nannochloropsis salina. Phytochemistry 2001, 56, 677–684. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Marty, J.-C.; Miquel, J.-C.; Volkman, J.K. Free radical oxidation (autoxidation) of alkenones and other microalgal lipids in seawater. Org. Geochem. 2006, 37, 354–368. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Volkman, J.K.; Prahl, F.G.; Wakeham, S.G. Biotic and abiotic degradation of alkenones and implications for paleoproxy applications: A review. Org. Geochem. 2013, 59, 95–113. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How effective are they to prevent age-related diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [Green Version]
- Fiedor, J.; Fiedor, L.; Haeßner, R.; Scheer, H. Cyclic Endoperoxides of β-Carotene, potential pro-oxidants, as products of chemical quenching of singlet oxygen. Biochim. Biophys. Acta—Bioenerg. 2005, 1709, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliman, M.; May, J.C.; McLean, J.A. Lipid analysis and lipidomics by structurally selective ion mobility-mass spectrometry. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2011, 1811, 935–945. [Google Scholar] [CrossRef] [Green Version]
























| ∆5-Sterols | [M − 143.0887]+ |
|---|---|
| 24-Nor-cholesta-5,22-dien-3β,6α/β-diols | 387.3084 |
| 24-Nor-cholest-5-en-3β,6α/β-diols | 389.3240 |
| Cholesta-5,22-dien-3β,6α/β-diols | 401.3240 |
| Cholesta-5,24-dien-3β,6α/β-diols | 401.3240 |
| Cholest-5-en-3β,6α/β-diols | 403.3396 |
| 24-Methylcholest-5-en-3β,6α/β-diols | 417.3562 |
| 24-Methylcholesta-5,22-dien-3β,6α/β-diols | 415.3396 |
| 24-Methylcholesta-5,24/28-dien-3β,6α/β-diols | 415.3396 |
| 24-Ethylcholest-5-en-3β,6α/β-diols | 431.3710 |
| 24-Ethylcholesta-5,22-dien-3β,6α/β-diols | 429.3552 |
| MUFAs | (OH-Position) m/z | (OH-Position) m/z | (OH-Position) m/z | (OH-Position) m/z |
|---|---|---|---|---|
| C16:1∆9 | (9-) 199.1518 a | (8-) 213.1675 a | (10-) 329.1968 b | (11-) 343.2125 b |
| C16:1∆11 | (11-) 171.1206 | (10-) 185.1363 | (12-) 357.2280 | (13-) 371.2437 |
| C18:1∆9 | (9-) 227.1830 | (8-) 241.1987 | (10-) 329.1968 | (11-) 343.2125 |
| C18:1∆11 | (11-) 199.1518 | (10-) 213.1675 | (12-) 357.2280 | (13-) 371.2437 |
| C20:1∆9 | (9-) 255.2139 | (8-) 269.2295 | (10-) 329.1968 | (11-) 343.2125 |
| C20:1∆11 | (11-) 227.1830 | (10-) 241.1987 | (12-) 357.2280 | (13-) 371.2437 |
| C22:1∆9 | (9-) 283.2451 | (8-) 297.2607 | (10-) 329.1968 | (11-) 343.2125 |
| C22:1∆11 | (11-) 255.2139 | (10-) 269.2295 | (12-) 357.2280 | (13-) 371.2437 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rontani, J.-F. Use of Gas Chromatography-Mass Spectrometry Techniques (GC-MS, GC-MS/MS and GC-QTOF) for the Characterization of Photooxidation and Autoxidation Products of Lipids of Autotrophic Organisms in Environmental Samples. Molecules 2022, 27, 1629. https://doi.org/10.3390/molecules27051629
Rontani J-F. Use of Gas Chromatography-Mass Spectrometry Techniques (GC-MS, GC-MS/MS and GC-QTOF) for the Characterization of Photooxidation and Autoxidation Products of Lipids of Autotrophic Organisms in Environmental Samples. Molecules. 2022; 27(5):1629. https://doi.org/10.3390/molecules27051629
Chicago/Turabian StyleRontani, Jean-François. 2022. "Use of Gas Chromatography-Mass Spectrometry Techniques (GC-MS, GC-MS/MS and GC-QTOF) for the Characterization of Photooxidation and Autoxidation Products of Lipids of Autotrophic Organisms in Environmental Samples" Molecules 27, no. 5: 1629. https://doi.org/10.3390/molecules27051629