Antibiotic Removal from the Aquatic Environment with Activated Carbon Produced from Pumpkin Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
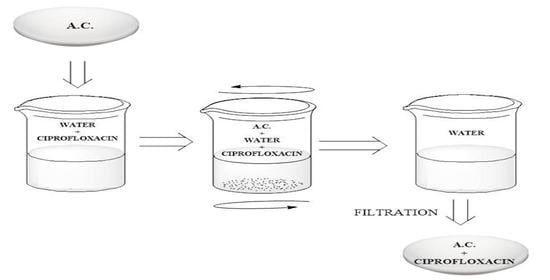
2.2. Preparation of the Adsorbent
2.3. Characterization of the Adsorbent
2.4. Adsorption Studies
3. Results
3.1. Characterization
3.2. Adsorption
3.3. Isotherm Studies
- Kc: Equilibrium constant
- Ca: Concentration of substance retained by the adsorbent (mg·L−1)
- Ce: Concentration of residual substance in solution (mg·L−1)
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Darweesh, T.M.; Ahmed, M.J. Adsorption of ciprofloxacin and norfloxacin from aqueous solution onto granular activated carbon in fixed bed column. Ecotoxicol. Environ. Saf. 2017, 138, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Khan, K.N.; Rasool, S.; Mustafa, G.; Saif-Ur-Rehman, M.; Nazar, M.F.; Sun, Q.; Yu, C.-P. Occurrence and ecological risk assessment of fluoroquinolone antibiotics in hospital waste of Lahore, Pakistan. Environ. Toxicol. Pharmacol. 2016, 42, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Pouretedal, H.; Sadegh, N. Effective removal of amoxicillin, cephalexin, tetracycline and penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J. Water Process Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Prutthiwanasan, B.; Phechkrajang, C.; Suntornsuk, L. Fluorescent labelling of ciprofloxacin and norfloxacin and its application for residues analysis in surface water. Talanta 2016, 159, 74–79. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500, 250–269. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Li, G.; Gao, B.; Yue, Q.; Li, X. Characterization and ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation. Bioresour. Technol. 2016, 217, 239–244. [Google Scholar] [CrossRef]
- Carabineiro, S.; Thavorn-Amornsri, T.; Pereira, M.; Serp, P.; Figueiredo, J. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal. Today 2012, 186, 29–34. [Google Scholar] [CrossRef]
- Zhu, L.; Santiago-Schübel, B.; Xiao, H.; Hollert, H.; Kueppers, S. Electrochemical oxidation of fluoroquinolone antibiotics: Mechanism, residual antibacterial activity and toxicity change. Water Res. 2016, 102, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Čvančarová, M.; Moeder, M.; Filipová, A.; Cajthaml, T. Biotransformation of fluoroquinolone antibiotics by ligninolytic fungi–Metabolites, enzymes and residual antibacterial activity. Chemosphere 2015, 136, 311–320. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Pretali, L.; Ferri, E.N.; Profumo, A. Sunlight-induced degradation of fluoroquinolones in wastewater effluent: Photoproducts identification and toxicity. Chemosphere 2015, 134, 313–318. [Google Scholar] [CrossRef]
- Feng, M.; Wang, X.; Chen, J.; Qu, R.; Sui, Y.; Cizmas, L.; Wang, Z.; Sharma, V.K. Degradation of fluoroquinolone antibiotics by ferrate (VI): Effects of water constituents and oxidized products. Water Res. 2016, 103, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour, B.; Yamini, Y.; Moradi, M. Application of ionic surfactant as a carrier and emulsifier agent for the microextraction of fluoroquinolones. J. Pharm. Biomed. Anal. 2012, 66, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gao, N.; Yang, Y.; Zhang, Y. Kinetics and transformation pathways on oxidation of fluoroquinolones with thermally activated persulfate. Chem. Eng. J. 2016, 292, 82–91. [Google Scholar] [CrossRef]
- Ferreira, V.R.; Amorim, C.L.; Cravo, S.M.; Tiritan, M.E.; Castro, P.M.; Afonso, C.M. Fluoroquinolones biosorption onto microbial biomass: Activated sludge and aerobic granular sludge. Int. Biodeterior. Biodegrad. 2016, 110, 53–60. [Google Scholar] [CrossRef]
- Tan, F.; Sun, D.; Gao, J.; Zhao, Q.; Wang, X.; Teng, F.; Quan, X.; Chen, J. Preparation of molecularly imprinted polymer nanoparticles for selective removal of fluoroquinolone antibiotics in aqueous solution. J. Hazard. Mater. 2013, 244, 750–757. [Google Scholar] [CrossRef]
- Depci, T.; Alkan, S.; Kul, A.; ÖNAL, Y.; Alacabey, I.; Dişli, E. Characteristic properties of adsorbed catalase onto activated carbon based adiyaman lignite. Fresenius Environ. Bull. 2011, 20, 2371–2378. [Google Scholar]
- Maraschi, F.; Sturini, M.; Speltini, A.; Pretali, L.; Profumo, A.; Pastorello, A.; Kumar, V.; Ferretti, M.; Caratto, V. TiO2-modified zeolites for fluoroquinolones removal from wastewaters and reuse after solar light regeneration. J. Environ. Chem. Eng. 2014, 2, 2170–2176. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Tarantino, S.; Gualtieri, A.F.; Zema, M. Removal of fluoroquinolone contaminants from environmental waters on sepiolite and its photo-induced regeneration. Chemosphere 2016, 150, 686–693. [Google Scholar] [CrossRef]
- Liang, Z.; Zhaob, Z.; Sun, T.; Shi, W.; Cui, F. Adsorption of quinolone antibiotics in spherical mesoporous silica: Effects of the retained template and its alkyl chain length. J. Hazard. Mater. 2016, 305, 8–14. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Fluoroquinolones antibiotics adsorption onto microporous activated carbon from lignocellulosic biomass by microwave pyrolysis. J. Taiwan Inst. Chem. Eng. 2014, 45, 219–226. [Google Scholar] [CrossRef]
- Almaz Kemal, K.S.; Michael, W.H. Adsorption of Cu (II) and Cd (II) onto Activated Carbon Prepared from Pumpkin Seed Shell. J. Environ. Sci. Pollut. Res. 2019, 5, 328–333. [Google Scholar] [CrossRef]
- Demiral, İ.; Şamdan, C.A. Preparation and characterisation of activated carbon from pumpkin seed shell using H3PO4. Anadolu Univ. J. Sci. Technol. A-Appl. Sci. Eng. 2016, 17, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Kuśmierek, K.; Świątkowski, A.; Dąbek, L. Removal of 2, 4, 6-trichlorophenol from aqueous solutions using agricultural waste as low-cost adsorbents. Environ. Prot. Eng. 2017, 43, 149–163. [Google Scholar] [CrossRef]
- Demiral, İ.; Bektaş, T.; Şamdan, C. Utilization of activated carbon prepared from pumpkin seed shell for the removal of dyestuff from aqueous solutions and wastewater by microwave radiation. Int. J. Sci. Technol. Res. 2019, 5, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Demiral, I.; Aydın Şamdan, C.; Demiral, H. Production and characterization of activated carbons from pumpkin seed shell by chemical activation with ZnCl2. Desalin. Water Treat. 2016, 57, 2446–2454. [Google Scholar] [CrossRef]
- Huang, G.G.; Liu, Y.-F.; Wu, X.X.; Cai, J.J. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. New Carbon Mater. 2019, 34, 247–257. [Google Scholar] [CrossRef]
- Le Van, K.; Thu, T.L.T.; Thu, H.N.T.; Van Hoang, H. Activated Carbon by KOH and NaOH Activation: Preparation and Electrochemical Performance in K2SO4 and Na2SO4 Electrolytes. Russ. J. Electrochem. 2019, 55, 900–907. [Google Scholar] [CrossRef]
- Cazedey, E.C.L.; Salgado, H.R.N. Spectrophotometric determination of ciprofloxacin hydrochloride in ophthalmic solution. Adv. Anal. Chem. 2012, 2, 74–79. [Google Scholar] [CrossRef]
- Erol, K.; Uzun, L. Two-step polymerization approach for synthesis of macroporous surface ion-imprinted cryogels. J. Macromol. Sci. Part A 2017, 54, 867–875. [Google Scholar] [CrossRef]
- Erol, K.; Bolat, M.; Tatar, D.; Nigiz, C.; Köse, D.A. Synthesis, characterization and antibacterial application of silver nanoparticle embedded composite cryogels. J. Mol. Struct. 2020, 1200, 127060. [Google Scholar] [CrossRef]
- Erol, K.; Tatar, D.; Veyisoğlu, A.; Tokatlı, A. Antimicrobial magnetic poly (GMA) microparticles: Synthesis, characterization and lysozyme immobilization. J. Polym. Eng. 2020, 41, 144–154. [Google Scholar] [CrossRef]
- Erol, K. Synthesis, Characterization and Chromatographic Applications of Antimicrobial Cryogels. Hacet. J. Biol. Chem. 2017, 45, 187–195. [Google Scholar] [CrossRef]
- Ece, M.Ş. Synthesis and characterization of activated carbon supported magnetic nanoparticles (Fe3O4/AC@SiO2@Sulfanilamide) and its application in removal of toluene and benzene. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126231. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Xia, H.; Duan, X. Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol. 2010, 101, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, J.; Lv, Z.; Wang, Q.; Ge, H.; Wang, X.; Hong, B. Preparation and utilization of cigarette filters based activated carbon for removal CIP and SDS from aqueous solutions. Chem. Phys. Lett. 2020, 747, 137343. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Wang, Z.; Lin, S. Removal of aqueous fluoroquinolones with multi-functional activated carbon (MFAC) derived from recycled long-root Eichhornia crassipes: Batch and column studies. Environ. Sci. Pollut. Res. 2019, 26, 34345–34356. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Guo, J.; Li, Y.; Zhang, H.; Zhu, J.; Xie, X. Enhanced adsorption of ciprofloxacin by KOH modified biochar derived from potato stems and leaves. Water Sci. Technol. 2018, 77, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Acet, Ö.; Baran, T.; Erdönmez, D.; Aksoy, N.H.; Alacabey, İ.; Menteş, A.; Odabaşi, M. O-carboxymethyl chitosan Schiff base complexes as affinity ligands for immobilized metal-ion affinity chromatography of lysozyme. J. Chromatogr. A 2018, 1550, 21–27. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- Banerjee, P.; Sau, S.; Das, P.; Mukhopadhayay, A. Optimization and modelling of synthetic azo dye wastewater treatment using graphene oxide nanoplatelets: Characterization toxicity evaluation and optimization using artificial neural network. Ecotoxicol. Environ. Saf. 2015, 119, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Wakkel, M.; Khiari, B.; Zagrouba, F. Basic red 2 and methyl violet adsorption by date pits: Adsorbent characterization, optimization by RSM and CCD, equilibrium and kinetic studies. Environ. Sci. Pollut. Res. 2019, 26, 18942–18960. [Google Scholar] [CrossRef] [PubMed]
- Erol, K. The adsorption of calmoduline via nicotinamide immobilized poly (HEMA-GMA) cryogels. J. Turk. Chem. Soc. Sect. A Chem. 2017, 4, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Erol, K. Polychelated cryogels: Hemoglobin adsorption from human blood. Artif. Cells Nanomed. Biotechnol. 2017, 45, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Erol, K.; Yıldız, E.; Alacabey, İ.; Karabörk, M.; Uzun, L. Magnetic diatomite for pesticide removal from aqueous solution via hydrophobic interactions. Environ. Sci. Pollut. Res. 2019, 26, 33631–33641. [Google Scholar] [CrossRef]
- Erol, K. DNA adsorption via Co (II) immobilized cryogels. J. Macromol. Sci. Part A 2016, 53, 629–635. [Google Scholar] [CrossRef]
- Erol, B.; Erol, K.; Gökmeşe, E. The effect of the chelator characteristics on insulin adsorption in immobilized metal affinity chromatography. Process Biochem. 2019, 83, 104–113. [Google Scholar] [CrossRef]
- Kireç, O.; Alacabey, İ.; Erol, K.; Alkan, H. Removal of 17β-estradiol from aqueous systems with hydrophobic microspheres. J. Polym. Eng. 2021, 41, 226–234. [Google Scholar] [CrossRef]
- Alacabey, İ.; Acet, Ö.; Önal, B.; Dikici, E.; Karakoç, V.; Gürbüz, F.; Alkan, H.; Odabaşı, M. Pumice particle interface: A case study for immunoglobulin G purification. Polym. Bull. 2021, 78, 5593–5607. [Google Scholar] [CrossRef]
- Satır Tosun, İ.; Erol, K. Calcined Eggshell for Removal of Victoria Blue R Dye from Wastewater Medium by Adsorption. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 47–56. [Google Scholar] [CrossRef]
- Czepirski, L.; Balys, M.R.; Komorowska-Czepirska, E. Some generalization of Langmuir adsorption isotherm. Internet J. Chem. 2000, 3, 1099–8292. [Google Scholar]
- Mittal, A.; Kurup, L.; Mittal, J. Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J. Hazard. Mater. 2007, 146, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alacabey, İ.; Kul, A.R.; Şakir, E.; Alkan, H. Van Gölü Doğal Sediment ve Modifiye Sediment Üzerine Krom (III) Adsorpsiyonu (İzoterm ve Termodinamik Analiz Çalışması). Dicle Üniversitesi Mühendislik Fakültesi Mühendislik Dergisi 2020, 11, 1225–1232. [Google Scholar] [CrossRef]
- Ece, M.S.A.; Kutluay, S.; Şahin, O.M.; Horoz, S. Development of novel Fe3O4/AC@SiO2@1, 4-DAAQ magnetic nanoparticles with outstanding VOC removal capacity: Characterization, optimization, reusability, kinetics, and equilibrium studies. Ind. Eng. Chem. Res. 2020, 59, 21106–21123. [Google Scholar] [CrossRef]
- Shin, H.S.; Kim, J.H. Isotherm, kinetic and thermodynamic characteristics of adsorption of paclitaxel onto Diaion HP-20. Process Biochem. 2016, 51, 917–924. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Naushad, M.; Ali, R. Kinetic, equilibrium isotherm and thermodynamic studies of Cr (VI) adsorption onto low-cost adsorbent developed from peanut shell activated with phosphoric acid. Environ. Sci. Pollut. Res. 2013, 20, 3351–3365. [Google Scholar] [CrossRef]
- Kiran, B.; Kaushik, A. Chromium binding capacity of Lyngbya putealis exopolysaccharides. Biochem. Eng. J. 2008, 38, 47–54. [Google Scholar] [CrossRef]
- Caliskan, N.; Kul, A.R.; Alkan, S.; Sogut, E.G.; Alacabey, I. Adsorption of Zinc (II) on diatomite and manganese-oxide-modified diatomite: A kinetic and equilibrium study. J. Hazard. Mater. 2011, 193, 27–36. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Ngah, W.W.; Fatinathan, S. Adsorption of Cu (II) ions in aqueous solution using chitosan beads, chitosan—GLA beads and chitosan—Alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Hu, X.J.; Wang, J.S.; Liu, Y.G.; Li, X.; Zeng, G.M.; Bao, Z.L.; Zeng, X.X.; Chen, A.W.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Sogut, E.G.; Caliskan, N. Removal of lead, copper and cadmium ions from aqueous solution using raw and thermally modified diatomite. Desalin. Water Treat. 2017, 58, 154–167. [Google Scholar] [CrossRef]







| Element Name | Amount |
|---|---|
| Nitrogen | 1.2331 |
| Carbon | 96.9629 |
| Hydrogen | 0.313 |
| Totals | 98.509 |
| Adsorbent | BET (m2·g−1) | qm (mg·g−1) | References |
|---|---|---|---|
| Albizia lebbeck seed pods | 1825 | 131.14 | [21] |
| Smoked cigarette filters (CFAC-5) | 2726 | 464.4 | [36] |
| Long-root Eichhornia crassipes | - | 145.00 | [37] |
| Potato stems and leaves (APB) | 38.75 | 23.36 | [38] |
| Pumpkin seeds | 2729.7 | 884.90 | This study |
| Isotherm | Linear Form | Temp. (K) | Constant Parameters | |||
|---|---|---|---|---|---|---|
| Freundlich | n | 1/n | KF (mg·g−1) | R2 | ||
| 288 | 4.6472 | 0.2152 | 265.58 | 0.8732 | ||
| 303 | 4.8094 | 0.2079 | 311.35 | 0.8902 | ||
| 318 | 5.7614 | 0.1736 | 418.46 | 0.9470 | ||
| Langmuir-1 | KL (L·mg−1) | qm (mg·g−1) | R2 | |||
| 288 | 0.0836 | 804.88 | 0.994 | |||
| 303 | 0.2781 | 819.61 | 0.9972 | |||
| 318 | 0.4176 | 884.90 | 0.9983 | |||
| Langmuir-2 | KL (L·mg−1) | qm (mg·g−1) | R2 | |||
| 288 | 0.0951 | 792.56 | 0.9537 | |||
| 303 | 0.7119 | 774.08 | 0.9598 | |||
| 318 | 1.0181 | 832.22 | 0.9502 | |||
| Langmuir-3 | KL (L·mg−1) | qm (mg·g−1) | R2 | |||
| 288 | 0.1013 | 784.18 | 0.8899 | |||
| 303 | 0.6941 | 777.83 | 0.9119 | |||
| 318 | 0.9797 | 838.99 | 0.8881 | |||
| Langmuir-4 | KL (L·mg−1) | qm (mg·g−1) | R2 | |||
| 288 | 0.0901 | 802.84 | 0.8899 | |||
| 303 | 0.6329 | 785.65 | 0.4683 | |||
| 318 | 0.8701 | 850.73 | 0.8881 | |||
| Langmuir-5 | KL (L·mg−1) | qm (mg·g−1) | R2 | |||
| 288 | 0.0894 | 804.280 | 0.9537 | |||
| 303 | 0.6790 | 778.930 | 0.9379 | |||
| 318 | 0.9588 | 839.610 | 0.9502 | |||
| Temkin | b (kJ·mol−1) | KT (L·mg−1) | R2 | |||
| 288 | 0.0190 | 2.9322 | 0.9043 | |||
| 303 | 0.0192 | 4.5938 | 0.9264 | |||
| 318 | 0.2220 | 22.1366 | 0.9754 | |||
| Dubinin–Radushkevich | qm (mg·g−1) | E (kJ·mol−1) | R2 | |||
| 288 | 717.21 | 0.1622 | 0.9424 | |||
| 303 | 780.25 | 0.2427 | 0.943 | |||
| 318 | 836.73 | 0.6597 | 0.8905 | |||
| Co | ΔH°, kJ·mol−1 | ΔS°, J·mol−1 | ΔG°, kJ·mol−1 | ||
|---|---|---|---|---|---|
| 288 K | 303 K | 313 K | |||
| 100 | 39.39 | 150.87 | −4.34 | −5.72 | −8.93 |
| 150 | 25.71 | 101.54 | −3.63 | −4.84 | −6.70 |
| 200 | 18.77 | 70.14 | −1.53 | −2.27 | −3.66 |
| 250 | 16.43 | 59.14 | −0.61 | −1.45 | −2.39 |
| 300 | 9.62 | 33.85 | −0.16 | −0.58 | −1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alacabey, İ. Antibiotic Removal from the Aquatic Environment with Activated Carbon Produced from Pumpkin Seeds. Molecules 2022, 27, 1380. https://doi.org/10.3390/molecules27041380
Alacabey İ. Antibiotic Removal from the Aquatic Environment with Activated Carbon Produced from Pumpkin Seeds. Molecules. 2022; 27(4):1380. https://doi.org/10.3390/molecules27041380
Chicago/Turabian StyleAlacabey, İhsan. 2022. "Antibiotic Removal from the Aquatic Environment with Activated Carbon Produced from Pumpkin Seeds" Molecules 27, no. 4: 1380. https://doi.org/10.3390/molecules27041380