Synthesis of Vitamin B3 through a Heterogeneous Photocatalytic Approach Using Metal-Free Carbon Nitride-Based Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the GCN-Based Photocatalysts
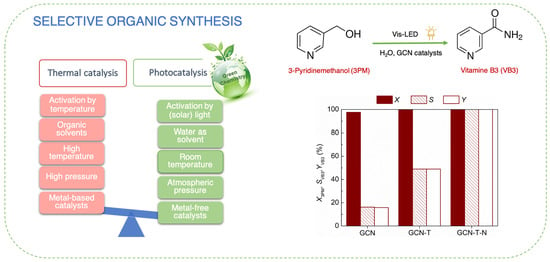
2.2. Photocatalytic Results
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Metal-Free GCN-Based Catalysts
3.3. Photocatalyst Characterization
3.4. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chuck, R. Technology development in nicotinate production. Appl. Catal. A Gen. 2005, 280, 75–82. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Pellagra and alcoholism: A biochemical perspective. Alcohol Alcohol. 2014, 49, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Spasiano, D.; Marotta, R.; Di Somma, I.; Mancini, G. Production of pyridinecarboxy aldehydes, nicotinic and isonicotinic and picolinic acids by TiO2-sacrificial photocatalysis at ambient conditions and in aqueous solution through artificial solar radiation. Appl. Catal. B Environ. 2015, 163, 248–257. [Google Scholar] [CrossRef]
- Yurdakal, S.; Çetinkaya, S.; Şarlak, M.B.; Özcan, L.; Loddo, V.; Palmisano, L. Photoelectrocatalytic oxidation of 3-pyridinemethanol to 3-pyridinemethanal and vitamin B3 by TiO2 nanotubes. Catal. Sci. Technol. 2020, 10, 124–137. [Google Scholar] [CrossRef]
- Yurdakal, S.; Yanar, Ş.Ö.; Çetinkaya, S.; Alagöz, O.; Yalçın, P.; Özcan, L. Green photocatalytic synthesis of vitamin B3 by Pt loaded TiO2 photocatalysts. Appl. Catal. B Environ. 2017, 202, 500–508. [Google Scholar] [CrossRef]
- Alfè, M.; Spasiano, D.; Gargiulo, V.; Vitiello, G.; Di Capua, R.; Marotta, R. TiO2/graphene-like photocatalysts for selective oxidation of 3-pyridine-methanol to vitamin B3 under UV/solar simulated radiation in aqueous solution at room conditions: The effect of morphology on catalyst performances. Appl. Catal. A Gen. 2014, 487, 91–99. [Google Scholar] [CrossRef]
- Sobahi, T.R.; Amin, M.S. Upgrading the photocatalytic achievement of g-C3N4 nanosheets along decoration with Ag@TiO2 nanospheres for the preparation of vitamin B3. Appl. Nanosci. 2019, 9, 1621–1636. [Google Scholar] [CrossRef]
- Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D. Heterogeneous photocatalytic organic synthesis: State-of-the-art and future perspectives. Green Chem. 2016, 18, 5391–5411. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; McMillan, P.F. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, L.; Xing, J.; Utama, M.I.B.; Lu, X.; Du, K.; Li, Y.; Hu, X.; Wang, S.; Genç, A.; et al. High-yield synthesis and optical properties of g-C3N4. Nanoscale 2015, 7, 12343–12350. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, E.S.; Moura, N.M.M.; Coutinho, A.; Dražić, G.; Teixeira, B.M.S.; Sobolev, N.A.; Silva, C.G.; Neves, M.G.P.M.S.; Prieto, M.; Faria, J.L. β-Cyclodextrin as a precursor to holey C-doped g-C3N4 nanosheets for photocatalytic hydrogen generation. ChemSusChem 2018, 11, 2681–2694. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Sampaio, M.J.; Faria, J.L.; Silva, C.G. Aqueous solution photocatalytic synthesis of p-anisaldehyde by using graphite-like carbon nitride photocatalysts obtained via the hard-templating route. RSC Adv. 2020, 10, 19431–19442. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.-Q.; Bao, S.-J.; Lu, S.; Xu, M.; Long, D.; Pu, S. Tuning and thermal exfoliation graphene-like carbon nitride nanosheets for superior photocatalytic activity. Ceram. Int. 2016, 42, 18521–18528. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Song, X.; Zhang, X.; Li, X.; Che, H.; Huo, P.; Ma, C.; Yan, Y.; Yang, G. Enhanced light utilization efficiency and fast charge transfer for excellent CO2 photoreduction activity by constructing defect structures in carbon nitride. J. Colloid Interface Sci. 2020, 578, 574–583. [Google Scholar] [CrossRef]
- Lopes, J.C.; Sampaio, M.J.; Fernandes, R.A.; Lima, M.J.; Faria, J.L.; Silva, C.G. Outstanding response of carbon nitride photocatalysts for selective synthesis of aldehydes under UV-LED irradiation. Catal. Today 2020, 357, 32–38. [Google Scholar] [CrossRef]
- Svoboda, L.; Praus, P.; Lima, M.J.; Sampaio, M.J.; Matýsek, D.; Ritz, M.; Dvorský, R.; Faria, J.L.; Silva, C.G. Graphitic carbon nitride nanosheets as highly efficient photocatalysts for phenol degradation under high-power visible LED irradiation. Mater. Res. Bull 2018, 100, 322–332. [Google Scholar] [CrossRef]
- Lima, M.J.; Silva, A.M.T.; Silva, C.G.; Faria, J.L. Graphitic carbon nitride modified by thermal, chemical and mechanical processes as metal-free photocatalyst for the selective synthesis of benzaldehyde from benzyl alcohol. J. Catal. 2017, 353, 44–53. [Google Scholar] [CrossRef]
- Lei, G.; Cao, Y.; Zhao, W.; Dai, Z.; Shen, L.; Xiao, Y.; Jiang, L. Exfoliation of graphitic carbon nitride for enhanced oxidative desulfurization: A facile and general strategy. ACS Sustain. Chem. Eng. 2019, 7, 4941–4950. [Google Scholar] [CrossRef]
- Dong, G.; Wen, Y.; Fan, H.; Wang, C.; Cheng, Z.; Zhang, M.; Ma, J.; Zhang, S. Graphitic carbon nitride with thermally-induced nitrogen defects: An efficient process to enhance photocatalytic H2 production performance. RSC Adv. 2020, 10, 18632–18638. [Google Scholar] [CrossRef]
- Sun, B.; Yu, H.; Yang, Y.; Li, H.; Zhai, C.; Qian, D.-J.; Chen, M. New complete assignment of X-ray powder diffraction patterns in graphitic carbon nitride using discrete Fourier transform and direct experimental evidence. Phys. Chem. Chem. Phys. 2017, 19, 26072–26084. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural investigation of graphitic carbon nitride via XRD and neutron diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Yang, Y.; Yin, L.-C.; Kang, X.; Liu, G.; Cheng, H.-M. An amorphous carbon nitride photocatalyst with greatly extended visible-light-responsive range for photocatalytic hydrogen generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Kong, X.Y.; Ng, B.-J.; Chew, Y.-H.; Mohamed, A.R.; Chai, S.-P. Midgap-state-mediated two-step photoexcitation in nitrogen defect-modified g-C3N4 atomic layers for superior photocatalytic CO2 reduction. Catal. Sci. Technol. 2019, 9, 2335–2343. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Lin, S.; Zhang, Y.; Wang, X. Activation of n → π* transitions in two-dimensional conjugated polymers for visible light photocatalysis. J. Phys. Chem. C 2014, 118, 29981–29989. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Sampaio, M.J.; Dražić, G.; Faria, J.L.; Silva, C.G. Efficient removal of parabens from real water matrices by a metal-free carbon nitride photocatalyst. Sci. Total Environ. 2019, 716, 135346. [Google Scholar] [CrossRef]
- Wu, P.; Wang, J.; Zhao, J.; Guo, L.; Osterloh, F.E. Structure defects in g-C3N4 limit visible light driven hydrogen evolution and photovoltage. J. Mater. Chem. A 2014, 2, 20338–20344. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 9780471720911. [Google Scholar]
- Dijkstra, M.F.J.; Michorius, A.; Buwalda, H.; Panneman, H.J.; Winkelman, J.G.M.; Beenackers, A.A.C. Comparison of the efficiency of immobilized and suspended systems in photocatalytic degradation. Catal. Today 2001, 66, 487–494. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tayade, R.J. New generation energy-efficient light source for photocatalysis: LEDs for environmental applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- He, P.; Tang, X.; Chen, L.; Xie, P.; He, L.; Zhou, H.; Zhang, D.; Fan, T. Patterned Carbon Nitride–Based Hybrid Aerogel Membranes via 3D Printing for Broadband Solar Wastewater Remediation. Adv. Funct. Mater. 2018, 28, 1801121. [Google Scholar] [CrossRef]




 ), and VB3 (◊) concentrations over 7 h reaction using immobilized GCN-T-N.
), and VB3 (◊) concentrations over 7 h reaction using immobilized GCN-T-N.
 ), and VB3 (◊) concentrations over 7 h reaction using immobilized GCN-T-N.
), and VB3 (◊) concentrations over 7 h reaction using immobilized GCN-T-N.
| Catalyst | SBET (m2 g−1) | C/N Ratio | FWHM (°) | d (nm) |
|---|---|---|---|---|
| GCN | 7 | 0.56 | 2.09 | 4.2 |
| GCN-T | 60 | 0.56 | 2.23 | 3.9 |
| GCN-T-N | 22 | 0.58 | 2.54 | 3.8 |
| Entry | Catalyst | Solvent | Atmosphere | Irradiation System | Reaction Time (h) | X (%) | S (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | TiO2 | Water | Nitrogen | High-pressure UV lamp | 0.75 | 30 | 87 | [3] |
| 2 | TiO2 nanotubes | Water | Air | Fluorescent lamp | n.i. | 15 | 35 | [4] |
| 3 | Pt/TiO2 | Water | Air | Fluorescent lamp | 3 | 15 | 45 | [5] |
| 4 | TiO2/graphene | Water | Nitrogen | Mercury lamp | 5 | 63 | 35 | [6] |
| 5 | Ag@TiO2/g-C3N4 | Water | Air | Halogen lamp | 9 | 100 | 100 | [7] |
| 6 | GCN | Water | Air | Vis-LED | 7 | 98 | 16 | Present study |
| 7 | GCN-T | 100 | 49 | |||||
| 8 | GCN-T-N | 100 | 100 | |||||
| 9 | GCN-T-N * | 71 | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, R.A.; Sampaio, M.J.; Faria, J.L.; Silva, C.G. Synthesis of Vitamin B3 through a Heterogeneous Photocatalytic Approach Using Metal-Free Carbon Nitride-Based Catalysts. Molecules 2022, 27, 1295. https://doi.org/10.3390/molecules27041295
Fernandes RA, Sampaio MJ, Faria JL, Silva CG. Synthesis of Vitamin B3 through a Heterogeneous Photocatalytic Approach Using Metal-Free Carbon Nitride-Based Catalysts. Molecules. 2022; 27(4):1295. https://doi.org/10.3390/molecules27041295
Chicago/Turabian StyleFernandes, Raquel A., Maria J. Sampaio, Joaquim L. Faria, and Cláudia G. Silva. 2022. "Synthesis of Vitamin B3 through a Heterogeneous Photocatalytic Approach Using Metal-Free Carbon Nitride-Based Catalysts" Molecules 27, no. 4: 1295. https://doi.org/10.3390/molecules27041295