Production and Digestibility Studies of β-Galactosyl Xylitol Derivatives Using Heterogeneous Catalysts of LacA β-Galactosidase from Lactobacillus Plantarum WCFS1
Abstract
:1. Introduction
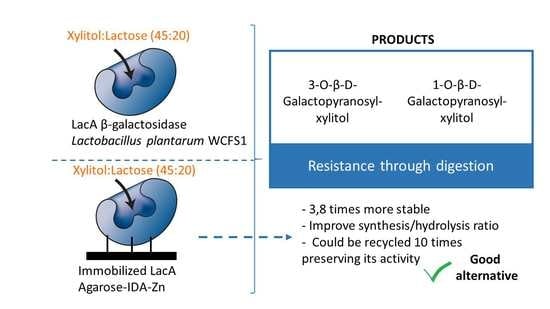
2. Results and Discussion
2.1. Immobilization of LacA β-Galactosidase on Different Supports
2.2. Immobilization and Purification of LacA β-Galactosidase in One Step
2.3. Synthesis of Galactosyl-Xylitol Derivatives Using Immobilized LacA β-Galactosidase
2.4. Reusability of Immobilized LacA β-Galactosidase
2.5. In Vitro Intestinal Digestion of β-Galactosyl Xylitol Derivatives Using Rat Small Intestinal Extract (RSIE)
3. Materials and Methods
3.1. Materials
3.2. Measure of the Enzyme Activity
3.3. Activation of the Different Supports
- (1)
- Activation of agarose with epoxy groups (17). 10 g of 10BCL agarose are suspended in 44 mL of distilled water, 16 mL of acetone, 3.28 g of NaOH, 0.2 g of NaBH4 and 11 mL of epichlorohydrin. The suspension was stirred for 16 h and then washed with plenty of water.
- (2)
- Modification of the agarose (Ag)- Epoxy support activated as described above with different Reactive Groups [30]. Triethylamine (TEA)-Ag supports: (Ag)-epoxide (TEA) was modified with 1M triethylamine in a 50% water-acetone solution for 24 h at a temperature of 20 °C. Iminodiacetic acid (IDA)—Ag-supports: Ag—epoxide was modified with 0.5 M iminodiacetic acid at pH 11 for 24 h at a temperature of 20 °C. Acid hydrolysis of agarose—Epoxide: 10 g of agarose-epoxide were suspended in 0.5 M hydrochloric acid and stirred for 1 h to hydrolyze the epoxide groups. Finally, all the supports were washed with water and dried under vacuum.
- (3)
- Oxidation of the diol groups of different supports [31]. A solution is prepared with 2 mL of NaIO4 0.1 M and 8 mL of distilled water and left under stirring for 90 min. Finally, the supports were washed with water and dried under vacuum. By this protocol Ag-IDA-CHO, Ag-TEA-CHO and monofunctional Ag-CHO were obtained.
- (4)
- Activation of the support Ag-IDA-Zn-CHO: The Ag-IDA-CHO agarose support was treated with 10 mL of a 30 mg/mL of ZnCl2 in water during 30 min and then washed with water.
- (5)
- Synthesis of monofunctional Ag-EDA-glutaraldehyde supports: 1 g of agarose previously activated with epoxy-groups, hydrolyzed with sulfuric acid and oxidized with sodium periodate, as described above, was aminated with 10 mL of 2M of ethylenediamine at pH 10 during 2 h and then the suspension was reduced with 10 mg per mL of sodium borohydride for 2 h. After this process, the support was washed with 1 M of NaCl and water [32]. Finally, 1 g of the aminated support was suspended in 1.7 mL of sodium phosphate 0.2 M pH 7 and 1.1 mL of a commercial glutaraldehyde solution 25%. This suspension was left under stirring during 16 h at 20 °C and then washed with water [33].
3.4. Immobilization of the Enzyme on the Different Supports
3.5. SDS-PAGE of Different Enzyme Preparations
3.6. Synthesis of β-Galactosyl Xylitol Derivatives with Soluble and Immobilized LacA β-Galactosidase
3.7. Reusability of Immobilized LacA β-Galactosidase
3.8. Characterization of Rat Small Intestinal Extract (RSIE)
3.8.1. Determination of the Protein Content
3.8.2. Determination of Main Hydrolytic Activities
3.8.3. In Vitro Digestion of Galactosyl Derivatives from Xylitol Using RSIE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. 2013. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA66/A66_R10-en.pdf (accessed on 21 September 2021).
- Livesey, G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr. Res. Rev. 2003, 16, 163–191. [Google Scholar] [CrossRef] [Green Version]
- Wölnerhanssen, B.K.; Meyer-Gerspach, A.C.; Beglinger, C.; Islam, M.S. Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review. Critl. Rev. Food Sci. 2019, 60, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Amo, K.; Arai, H.; Uebanso, T.; Fukaya, M.; Koganei, M.; Sasaki, H.; Yamamoto, H.; Taketani, E.; Takeda, E. Effects of xylitol on metabolic parameters and visceral fat accumulation. J. Clin. Biochem. Nutr. 2011, 49, 1–7. [Google Scholar]
- Zhan, L.; Cheng, J.; Chang, P.; Ngo, M.; DenBesten, P.; Hoover, C.; Featherstone, J.B.D. Effects of Xylitol Wipes on Cariogenic Bacteria and Caries in Young Children. J. Dent. Res. 2012, 91, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuncer, D.; Onen, A.; Yazici, A. Effect of chewing gums with xylitol, sorbitol and xylitol-sorbitol on the remineralization and hardness of initial enamel lesions in situ. Dent. Res. J. 2017, 11, 537–543. [Google Scholar]
- Asano, T.; Levitt, M.D.; Goetz, F.C. Xylitol absorption in healthy men. Diabetes 1973, 22, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food Sci. 2015, 55, 1514–1528. [Google Scholar] [CrossRef]
- Salminen, S.; Salminen, E.; Koivistoinen, P.; Bridges, J.; Marks, V. Gut microflora interactions with xylitol in the mouse, rat and man. Food Chem. Toxicol. 1985, 23, 985–990. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.; Ye, K.; Li, M.; Ying, J.; Wang, H.; Han, J.; Shi, L.; Xiao, J.; Shen, Y.; Feng, X.; et al. Xylitol enhances synthesis of propionate in the colon via cross-feeding of gut microbiota. Microbiome 2021, 9, 1–21. [Google Scholar] [CrossRef]
- Rice, T.; Zannini, E.K.; Arendt, E.; Coffey, A. A review of polyols—Biotechnological production, food applications, regulation, labeling and health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2034–2051. [Google Scholar] [CrossRef] [PubMed]
- Mahadevaiah, S.; Basavaiah, R.; Parida, M.; Batra, H.V. Optimal production of β-galactosidase from lactobacillus fermentum for the synthesis of prebiotic galactooligosaccharides (GOS). J. Pure Appl. Microbiol. 2020, 14, 2769–2780. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Manera, A.P.; Ores, J.C.; Sala, L.; Maugeri, F.; Kalil, S.-J. Kinetics and thermal properties of crude and purified β-galactosidase with potential for the production of galactooligosaccharides. Food Technol. Biotechnol. 2013, 51, 45–52. [Google Scholar]
- Delgado-Fernandez, P.; Plaza-Vinuesa, L.; Lizasoain-Sanchez, S.; de las Rivas, B.; Muñoz, R.; Jimeno, M.L.; García-Doyagüez, E.; Moreno, F.J.; Corzo, N. Hydrolysis of Lactose and Transglycosylation of Selected Sugar Alcohols by LacA β-Galactosidase from Lactobacillus plantarum WCFS1. J. Agric. Food Chem. 2020, 68, 7040–7050. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Mateo, C.; Bolivar, J.M.; Godoy, C.; Rocha-Martin, J.; Pessela, B.C.C.; Curiel, J.A.; Muñoz, R.; Guisan, J.M.; Fernandez-Lorente, G. Improvement of enzyme properties with a two-step Immobilization process on novel heterofunctional supports. Biomacromolecules 2010, 11, 3112–3117. [Google Scholar] [CrossRef] [Green Version]
- Neri, D.F.; Balcão, V.M.; Costa, R.S.; Rocha, I.C.; Ferreira, E.M.; Torres, D.P.; Rodríguez, L.R.M.; Carvalho Jr, L.B.; Teixeira, J.A. Galacto-oligosaccharides production during lactose hydrolysis by free Aspergillus oryzae β-galactosidase and immobilized on magnetic polysiloxane-polyvinyl alcohol. Food Chem. 2009, 115, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Cardelle-Cobas, A.; Olano, A.; Irazoqui, G.; Giacomini, C.; Batista-Viera, F.; Corzo, N.; Corzo-Martínez, M. Synthesis of oligosaccharides derived from lactulose (OsLu) using soluble and immobilized Aspergillus oryzae β-galactosidase. Front. Bioeng. Biotechnol. 2016, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Pham, M.L.; Leister, T.; Nguyen, H.A.; Do, B.C.; Pham, A.T.; Haltrich, D.; Yamabhai, M.; Nguyen, T.H.; Nguyen, T.T. Immobilization of β-galactosidases from Lactobacillus on chitin using a chitin-binding domain. J. Agric. Food Chem. 2017, 65, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.L.; Tran, A.M.; Kittibunchakul, S.; Nguyen, T.T.; Mathiesen, G.; Nguyen, T.H. Immobilization of β-galactosidases on the Lactobacillus cell surface using the peptidoglycan-binding motif LysM. Catalysts 2019, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Juśkiewicz, J.; Zduńczyk, Z.; Klewicki, R.; Gomez-Villalva, E. Physiological effects of dietary inulin, xylitol and β-galactosyl-derivatives of sugar alcohols in rat. Acta Aliment. 2004, 33, 303–311. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.; Olano, A.; Rastall, R.A.; Moreno, F.J. In vitro Digestibility of Dietary Carbohydrates: Toward a Standardized Methodology Beyond Amylolytic and Microbial Enzymes. Front. Nutr. 2019, 6, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira-Lazarte, A.; Olano, A.; Villamiel, M.; Moreno, F.J. Assessment of in vitro digestibility of dietary carbohydrates using rat small intestinal extract. J. Agric. Food Chem. 2017, 65, 8046–8053. [Google Scholar] [CrossRef]
- Delgado-Fernandez, P.; de Las Rivas, B.; Muñoz, R.; Jimeno, M.L.; Doyagüez, E.G.; Corzo, N.; Moreno, F.J. Biosynthesis of Nondigestible Galactose-Containing Hetero-oligosaccharides by Lactobacillus plantarum WCFS1 MelA α-Galactosidase. J. Agric. Food Chem. 2021, 69, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Shinoki, A.; Hara, H. Melibiose, a nondigestible disaccharide, promotes absorption of quercetin glycosides in rat small intestine. J. Agric. Food Chem. 2016, 64, 9335–9341. [Google Scholar] [CrossRef] [PubMed]
- Juśkiewicz, J.; Klewicki, R.; Zduńczyk, Z. Consumption of galactosyl derivatives of polyols beneficially affects cecal fermentation and serum parameters in rats. Nutr. Res. 2006, 26, 531–536. [Google Scholar] [CrossRef]
- Klewicki, R.; Klewicka, E. Antagonistic activity of lactic acid bacteria as probiotics against selected bacteria of the Enterobaceriacae family in the presence of polyols and their galactosyl derivatives. Biotechnol. Lett. 2004, 26, 317–320. [Google Scholar] [CrossRef]
- Mateo, C.; Fernández-Lorente, G.; Abian, O.; Fernández-Lafuente, R.; Guisán, J.M. Multifunctional epoxy-supports: A new tool to improve the covalent immobilization of proteins. The promotion of physical adsorptions of proteins on the supports before their covalent linkage. Biomacromolecules 2000, 1, 739–745. [Google Scholar] [CrossRef]
- Guisan, J.M. Aldehyde-agarose gels as activated supports for immobilization-stabilization of enzymes. Enzyme Microb. Technol. 1988, 10, 375–382. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Rosell, C.M.; Rodriguez, V.; Santana, C.; Soler, G.; Bastida, A.; Guisán, J.M. Preparation of activated supports containing low pK amino groups.A new tool for protein immobilization via the carboxyl coupling method. Enzyme Microb. Technol. 1993, 15, 546–550. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions. Enzyme Microb. Technol. 2006, 39, 877–882. [Google Scholar] [CrossRef]
- Laemmli, U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Sanz, M.L.; Villamiel, M.; Martinez-Castro, I. Inositols and carbohydrates in different fresh fruit juices. Food Chem. 2004, 87, 325–328. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ghazi, I.; Gomez De Segura, A.; Fernandez-Arrojo, L.; Alcalde, M.; Yates, M.; Rojas-Cervantes, M.L.; Plou, F.J.; Ballesteros, A. Immobilisation of fructosyltransferase from Aspergillus aculeatus on epoxy-activated Sepabeads EC for the synthesis of fructo-oligosaccharides. J. Mol. Catal. B-Enzyme 2005, 35, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Asare-Brown, E.; Bullock, C. Simple practical investigations using invertase. Biochem. Educ. 1988, 16, 98–100. [Google Scholar] [CrossRef]





| Support | Immobilized Enzyme (%) | Conserved Activity after Immobilization (%) | Final Activity after Reduction (%) |
|---|---|---|---|
| IDA-Zn-CHO (pH 7) | 100% | 100% | 85% |
| IDA-Zn-CHO (pH 7,8) | 100% | 100% | 85% |
| IDA-Zn-CHO (pH 7) (red. pH 8,5 Galactose) | 100% | 100% | 78% |
| IDA-Zn-CHO (pH 7) (red. pH 10) | 100% | 100% | 0% |
| Glyoxyl [CHO] (pH 7,8) | 75% | 25% | 13% |
| TEA-CHO (pH 7,8) | 100% | 25% | 2% |
| EDA-Glutaraldehyde (pH 7) | 100% | 100% | ----- |
| IDA-CHO (pH 7) | 0% | ----- | ----- |
| Imidazol (mM) | Non Adsorbed β-Gal (%) | Adsorbed Proteins (%) |
|---|---|---|
| 0 | 9 | 64.7 |
| 10 | 30 | 32.7 |
| 20 | 37 | 19.4 |
| 30 | 38 | 17.5 |
| LacA β-Galactosidase | Time (h) | Concentration (g L−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Xylitol | Galactose | Glucose | Lactose | Galactosyl Xylitol 1 a | Galactosyl Xylitol 2 b | GOS-Di c | GOS-Tri d | ||
| Soluble | 0 | 436.1 ± 5.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 236.6 ± 4.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 6 | 401.6 ± 10.6 | 4.4 ± 0.3 | 13.6 ± 0.1 | 123.3 ± 4.4 | 6.2 ± 0.1 | 24.3 ± 0.1 | 1.5 ± 0.1 | 0.9 ± 0.0 | |
| 24 | 398.6 ± 17.6 | 17.0 ± 0.7 | 71.3 ± 3.4 | 80.8 ± 0.4 | 12.1 ± 2.0 | 38.1 ± 1.3 | 1.9 ± 0.0 | 1.3 ± 0.1 | |
| 32 | 388.4 ± 23.1 | 17.3 ± 0.2 | 74.9 ± 0.8 | 58.3 ± 4.3 | 13.1 ± 0.1 | 39.1 ± 2.5 | 3.7 ± 0.0 | 2.0 ± 0.0 | |
| 48 | 383.7 ± 11.1 | 23.6 ± 2.4 | 89.2 ± 8.6 | 39.4 ± 0.5 | 13.7 ± 1.2 | 44.8 ± 1.6 | 6.4 ± 0.2 | 4.2 ± 0.2 | |
| Immobilized | 0 | 442.2 ± 3.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 205.4 ± 5.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 6 | 416.2 ± 0.8 | 5.1 ± 0.1 | 29.3 ± 0.6 | 176.2 ± 9.7 | 8.7 ± 0.1 | 30.8 ± 2.1 | 1.4 ± 0.2 | 2.3 ± 0.1 | |
| 24 | 397.0 ± 1.1 | 16.5 ± 0.0 | 57.7 ± 0.2 | 65.5 ± 0.3 | 10.8 ± 0.6 | 50.9 ± 0.4 | 3.3 ± 0.2 | 4.3 ± 0.3 | |
| 32 | 377.5 ± 3.3 | 21.1 ± 1.4 | 66.5 ± 4.1 | 47.1 ± 1.9 | 11.2 ± 0.5 | 52.3 ± 2.7 | 5.0 ± 0.0 | 4.5 ± 0.2 | |
| 48 | 368.1 ± 7.8 | 29.5 ± 0.3 | 71.3 ± 5.9 | 30.4 ± 2.7 | 14.1 ± 0.4 | 53.6 ± 3.3 | 7.6 ± 0.0 | 5.0 ± 0.3 | |
| Time (min) | Hydrolysis Degree a (%) | ||
|---|---|---|---|
| Galactosyl Xylitol 1 b | Galactosyl Xylitol 2 c | Lactose | |
| 15 | 1.1 ± 0.03 a | 5.5 ± 0.2 a | 8.5 ± 0.1 a |
| 30 | 1.8 ± 0.04 b | 8.2 ± 0.1 b | 32.2 ± 0.2 b |
| 60 | 2.1 ± 0.1 c | 14.0 ± 0.1 c | 51.1 ± 0.5 c |
| 90 | 2.7 ± 0.04 d | 15.1 ± 0.2 d | 54.1 ± 0.2 d |
| 120 | 6.0 ± 0.04 e | 15.5 ± 0.4 d | 55.5 ± 0.4 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosado, E.; Delgado-Fernández, P.; de las Rivas, B.; Muñoz, R.; Moreno, F.J.; Corzo, N.; Mateo, C. Production and Digestibility Studies of β-Galactosyl Xylitol Derivatives Using Heterogeneous Catalysts of LacA β-Galactosidase from Lactobacillus Plantarum WCFS1. Molecules 2022, 27, 1235. https://doi.org/10.3390/molecules27041235
Rosado E, Delgado-Fernández P, de las Rivas B, Muñoz R, Moreno FJ, Corzo N, Mateo C. Production and Digestibility Studies of β-Galactosyl Xylitol Derivatives Using Heterogeneous Catalysts of LacA β-Galactosidase from Lactobacillus Plantarum WCFS1. Molecules. 2022; 27(4):1235. https://doi.org/10.3390/molecules27041235
Chicago/Turabian StyleRosado, Eduardo, Paloma Delgado-Fernández, Blanca de las Rivas, Rosario Muñoz, Francisco Javier Moreno, Nieves Corzo, and Cesar Mateo. 2022. "Production and Digestibility Studies of β-Galactosyl Xylitol Derivatives Using Heterogeneous Catalysts of LacA β-Galactosidase from Lactobacillus Plantarum WCFS1" Molecules 27, no. 4: 1235. https://doi.org/10.3390/molecules27041235