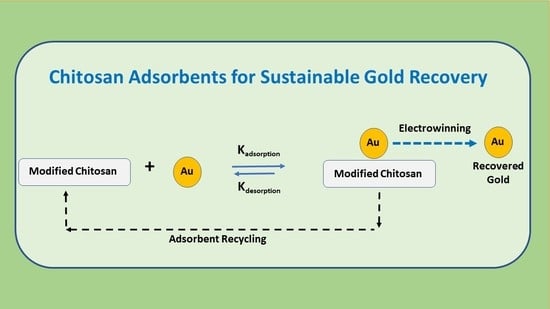
An Overview of Modified Chitosan Adsorbents for the Removal of Precious Metals Species from Aqueous Media
Abstract
:1. Introduction
2. Physical Modification of Chitosan
3. Covalently Grafted Chitosan
3.1. N-Containing Chitosan Derivatives
3.2. O-Containing Chitosan Derivatives
| Adsorbent | Characterization Methods | Metal Ions | Uptake Qm (mg/g) | Conditions | Literature Refs. | |||
|---|---|---|---|---|---|---|---|---|
| pH | T (K) | Time (h) | Dosage (g/L) | |||||
| GMCCR | FTIR, SEM, BET | Au(III), Pt(IV), Pd(II) | 169 (Au), 122 (Pt), 120 (Pd) | 1–4 | 298 | 24 | 3.33 | [35] |
| PAA-CS | FTIR | Gold cyanide | 10.18 | 7 | 298 | 24 | 1 | [36] |
| LMCCR | FTIR, BET, SEM | Au(III), Pt(IV), Pd(II) | 70 (Au), 109 (Pd), 129 (Pt) | 2 | 303, 313, 323 | 0.1–7 | 0.01–0.4 | [37] |
3.3. S-Containing Chitosan Derivatives

| Adsorbent | Characterization Methods | Metal Ions | Uptake Qm (mg/g) | Conditions | Literature Refs. | |||
|---|---|---|---|---|---|---|---|---|
| pH | T (K) | Time (h) | Dosage (g/L) | |||||
| CDF-CS | FTIR, SEM, XPS, and Zeta-potential | Au(III) | 478 | 1–10 | 298, 308, 318 | 6 | 0.4 | [41] |
| ETSA | FTIR, XPS, XRD, SEM | Au(III) | 6 | 1–3 | 303, 313, 323 | 25 | 0.2 | [43] |
| Thiourea GCC | SEM, | Au(III) | 5.0 | 1–4 | 298, 308, 318, 328 | 12 | 1.00 | [44] |
| TSC-CCB | FTIR, SEM, XPS, Zeta-potential | Au(III) | 1470 | 6 | 298 | 3–24 | 0.4 | [45] |
| Thiocarbamoyl Chitosan | XPS, 13C NMR, FTIR, Elemental analysis | Au(III), Pt(IV), Pd(II) | 0.8(Pt), 2.1(Pd), 2.3(Au) | 2 | 298 | 18 | 0.050 | [46] |
| CS-MoS2 | SEM, EDS, FTIR, XRD, XPS, and Zeta-potential | Au(III) | 3108 | 1–9 | 303, 314, 323 | 6 | 0.4 | [47] |
| CS-GTU | SEM, EDS, XRD, XPS | Au(III) | 741 | 1–9 | 303, 313, 323 | 30 | 0.4 | [48] |
3.4. Other Chitosan and Biopolymer Derivatives
| Adsorbent | Characterization Methods | Metal Ions | Uptake Qm (mg/g) | Conditions | Literature Refs. | |||
|---|---|---|---|---|---|---|---|---|
| pH | T (K) | Time (h) | Dosage (g/L) | |||||
| Chitosan | FTIR | Au(III) | 33 | 2 | 298 | 0.5 | 1.0 | [31] |
| Chitosan | FTIR, XPS | Pd(II) | n/a | 0.5–6 | 298 | 0.5 | 0.2 | [52] |
| Activated carbon (AC) | SEM, PSD | Au(III) | 67 | 1–11 | 298–348 | 6 | 0.03–0.18 | [53] |
| AC | SEM-EDS, FTIR, XPS | Au(I) | 25.9 | 6–10 | 308, 318, 328 | 3–50 | 0.05 | [54] |
| Modified AC | pHpzc, SEM, XPS, BET | Au(III) | 3.55 | 8.5 | 298, 308, 318 | 25 | 1.00 | [55] |
4. Conditions Influencing Batch Adsorption of Precious Metals
4.1. Temperature
4.2. Solution pH
4.3. Adsorption Capacity and the Number of Binding Sites of Adsorbents
4.4. Initial Source Concentration of Precious Metals
4.5. Competing Ions in the Precious Metal Matrix
4.6. Mixing Rate and Contact Time
5. Mechanism of Adsorption
5.1. Physical Sorption Mechanism
5.2. Chemisorption Mechanism
6. Desorption and Recovery of Precious Metals
7. Strategies for Enhancing the Adsorption Capacity
7.1. Pretreatment to Optimize Conditions of the Process
7.2. Increasing the Active Sites of the Chitosan Composites
7.3. Overview of the Solid-Liquid Extraction of Gold Ions by AC and Modified Chitosan Sorbents
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Management 2016, 57, 64–90. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Wilson, L.D.; Sammynaiken, R. Sorptive uptake studies of an aryl-arsenical with iron oxide composites on an activated carbon support. Materials 2014, 7, 1880–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, D.; Wilson, L.D. Synthesis and characterization of cellulose-geothite composites and their adsorption properties with roxarsone. Carbohydr. Polym. 2017, 169, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Chen, Y.; Zi, F.; GHu, X.; Qin, X.; Zhang, Y.; Tang, P.; He, Y.; He, P.; et al. Modification of activated carbon by chemical vapour deposition through thermal decomposition of thiourea for enhanced adsorption of gold thiosulfate complex. Sep. Purif. Technol. 2020, 241, 116632. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Self-Assembled and Cross-Linked Animal and Plant-Based Polysaccharides: Chitosan-Cellulose Composites and Their Anion Uptake Properties. ACS Appl. Mater. Interfaces 2016, 8, 33197–33209. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Torri, G.; Lichtfouse, E.; Kyzas, G.; Wilson, L.; Crini, N. Dyr removal by biosorption using cross-linked chitosan-based hydrogels. Environ. Chem. Lett. 2019, 17, 1645–1666. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.H. Gold(III) recovery from aqueous solutions by raw and modified chitosan: A review. Carbohydr. Polym. 2021, 256. [Google Scholar] [CrossRef]
- Wilson, L.D.; Tewari, B.B. Chitosan-based adsorbents: Environmental applications for the removal of arsenicals. In Chitosan-Based Adsorbents for Wastewater Treatment; Nasar, A., Ed.; Materials Research Forum: Millersville, PA, USA, 2018; Volume 34, pp. 133–160. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Crini, N.M. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Steiger, B.G.K.; Wilson, L.D. Modular Chitosan-Based Adsorbents for Tunable Uptake of Sulfate from Water. Int. J. Mol. Sci. 2020, 21, 7130. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Xue, C.; Wilson, L.D. Design and characterization of chitosan-based composite particles with tunable interfacial properties. Carbohydr. Polym. 2015, 132, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.D.; Xue, C. Macromolecular sorbent materials for urea capture. J. Appl. Polym. Sci. 2013, 128, 667–675. [Google Scholar] [CrossRef]
- Van der Lubben, I.M.; Verhoef, J.C.; van Aelst, A.C.; Borchard, G.; Junginger, H.E. Chitosan microparticles for oral vaccination: Preparetion, characterization and preliminary in vivo uptake studies in murine Peyer’s patches. Biomaterials 2001, 22, 687–694. [Google Scholar] [CrossRef]
- Guibal, E.; Milot, C.; Tobin, J.M. Metal-Anion Sorption by Chitosan Beads: Equilibrium and Kinetic Studies. Ind. Eng. Chem. Res. 1998, 37, 1451–1463. [Google Scholar] [CrossRef]
- Nuryono, N.; Miswanda, D.; Sakti, S.C.W.; Rusdiarso, B.; Krisbiantoro, P.A.; Utami, N.; Otomo, R.; Kamiya, Y. Chitosan-functionalized natural magnetic particle@silica modified with (3-chloropropyl) trimethoxysilane as a highly stable magnetic adsorbent for gold(III) ion. Mater. Chem. Phys. 2020, 255, 11. [Google Scholar] [CrossRef]
- Modrzejewska, Z.; Kaminski, W. Separation of Cr (VI) on Chitosan Membranes. Ind. Eng. Chem. Res. 1999, 38, 4946–4950. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Wu, Y.B.; Lee, S.T.; Shyong, J.Y.; Huang, R.N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Zhuang, S.T.; Zhu, K.K.; Wang, J.L. Fibrous chitosan cellulose composite as an efficient adsorbent for Co(II) removal. J. Clean. Prod. 2021, 285, 10. [Google Scholar] [CrossRef]
- Bilican, I.; Pekdemir, S.; Onses, M.S.; Akyuz, L.; Altuner, E.M.; Koc-Bilican, B.; Zang, L.-S.; Mujtaba, M.; MulercÌŒikas, P.; Kaya, M. Chitosan Loses Innate Beneficial Properties after Being Dissolved in Acetic Acid: Supported by Detailed Molecular Modeling. ACS Sustain. Chem. Eng. 2020, 8, 18083–18093. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wilson, L.D. Phosphate uptake studies of cross-linked chitosan bead materials. J. Colloid Interface Sci. 2017, 485, 201–212. [Google Scholar] [CrossRef]
- Bui, T.H.; Lee, W.; Jeon, S.B.; Kim, K.W.; Lee, Y. Enhanced Gold(III) adsorption using glutaraldehyde-crosslinked chitosan beads: Effect of crosslinking degree on adsorption selectivity, capacity, and mechanism. Sep. Purif. Technol. 2020, 248, 116989. [Google Scholar] [CrossRef]
- Park, S.-I.; Kwak, I.S.; Won, S.W.; Yun, Y.-S. Glutaraldehyde-crosslinked chitosan beads for sorptive separation of Au (III) and Pd (II): Opening a way to design reduction-coupled selectivity-tunable sorbents for separation of precious metals. J. Harzard. Mater. 2013, 248, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.H.; Jeon, S.; Lee, Y. Facile recovery of gold from e-waste by integrating chlorate leaching and selective adsorption using chitosan-based bioadsorbent. J. Environ. Chem. Eng. 2021, 9, 9. [Google Scholar] [CrossRef]
- Solgi, M.; Tabil, L.G.; Wilson, L.D. Modified biopolymer adsorbents for column treatment of sulfate species in saline aquifers. Materials 2020, 13, 2408. [Google Scholar] [CrossRef]
- Pestov, A.; Bratskaya, S. Chitosan and its derivatives as highly efficient polymer ligands. Molecules 2016, 21, 330. [Google Scholar] [CrossRef] [Green Version]
- Azarova, Y.A.; Pestov, A.V.; Ustinov, A.Y.; Bratskaya, S.Y. Application of chitosan and its N-heterocyclic derivatives forpreconcentration of noble metal ions and their determination usingatomic absorption spectrometry. Carbohydr. Polym. 2015, 134, 680–686. [Google Scholar]
- Wang, H.; Bao, C.; Li, F. Preparation and application of 4-amino-40-nitro azobenzene modified chitosan as a selective adsorbent for the determination of Au (III) and Pd (II). Microchim. Acta. 2010, 168, 99–105. [Google Scholar] [CrossRef]
- Liang, Y.-L.; Jin, C.; Hu, J.-T.; Liu, Z.; Li, Y.; Zeng, J.-R.; Wu, G.-Z.; Wei, X.-J. A highly efficient pathway to recover gold from acid aqueous solution by using an amidoxime-functionalized UHMWPE fiber. Rare Met. 2019, 38, 1105–1112. [Google Scholar] [CrossRef]
- Liu, F.L.; Hua, S.; Zhou, L.; Hu, B.W. Development and characterization of chitosan functionalized dialdehyde viscose fiber for adsorption of Au(III) and Pd(II). Int. J. Biol. Macromol. 2021, 173, 457–466. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Liang, K.H. Adsorption of Gold (III) Ions onto Chitosan and N-Carboxymethyl Chitosan: Equilibrium Studies. Ind. Eng. Chem. Res. 1999, 38, 1411–1414. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, Y.; Wang, Y. Preparation and adsorption properties of metal ions of crosslinked chitosan azacrown ethers. J. Appl. Polym. Sci. 1999, 74, 3053–3058. [Google Scholar] [CrossRef]
- Azarova, Y.A.; Pestov, A.V.; Bratskaya, S.Y. Application of chitosan and its derivatives for solid-phase extraction of metal and metalloid ions: A mini-review. Cellulose 2016, 23, 2273–2289. [Google Scholar] [CrossRef]
- Pang, L.J.; Li, R.; Hu, J.T.; Zhang, L.J.; Zhang, M.X.; Yang, C.G.; Wu, G.Z. Functionalized polyethylene fibers for the selective capture of palladium ions from aqueous solution. Appl. Surf. Sci. 2018, 433, 116–124. [Google Scholar] [CrossRef]
- Ramesh, A.; Hasegawa, H.; Sugimoto, W.; Maki, T.; Ueda, K. Adsorption of gold (III), platinum (IV) and palladium (II) onto glycine modified crosslinked chitosan resin. Bioresour. Technol. 2008, 99, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Swantomo, D.; Faturrahman, I.R.; Basuki, K.T.; Wongsawaeng, D. Chitosan-polyacrylamide graft copolymers prepared with gamma irradiation for gold cyanide adsorption. Polym.-Plast. Technol. Mater. 2020, 59, 1284–1291. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ramesh, A.; Maki, T.; Hasegawa, H.; Ueda, K. Adsorption of platinum (IV), palladium (II) and gold (III) from aqueous solutions onto l-lysine modified crosslinked chitosan resin. J. of Hazard. Mater. 2007, 146, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Arguelles-Monal, W.; Peniche-Covas, C. Preparation and characterization of a mercaptan derivative of chitosan for the removal of mercury from brines. Macromol. Chem. Phys. 1993, 207, 1–8. [Google Scholar]
- Guibal, E.; Milot, C.; Eterradossi, O.; Gauffier, C.; Domard, A. Study of molybdate ion sorption on chitosan gel beads by different spectrometric analyses. Inter. J. Biol. Macromol. 1999, 24, 49–59. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Fabrication of Au(III) ion-imprinted polymer based on thiol-modified chitosan. Int. J. Biol. Macromol. 2017, 105, 777–787. [Google Scholar] [CrossRef]
- Zhao, M.H.; Zhao, J.L.; Huang, Z.; Wang, S.X.; Zhang, L.B. One pot preparation of magnetic chitosan-cystamine composites for selective recovery of Au(III) from the aqueous solution. Int. J. Biol. Macromol. 2019, 137, 721–731. [Google Scholar] [CrossRef]
- Maity, S.; Naskar, N.; Jana, B.; Lahiri, S.; Ganguly, J. Fabrication of thiophene-chitosan hydrogel-trap for efficient immobilization of mercury (II) from aqueous environs. Carbohydr. Polym. 2021, 251, 116999. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Y.; Zhao, Y. Biosorption and reduction of Au (III) to gold nanoparticles by thiourea modified alginate. Carbohydr. Polym. 2017, 159, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, H.; Liu, S.; Yu, H.; Li, P.; Xing, R. Adsorption properties of gold onto a chitosan derivative. Inter. J. Biol. Macromol. 2012, 51, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Wang, S.X.; Zhang, L.B.; Hu, T.; Peng, J.H.; Cheng, S.; Fu, L.K. Synthesis and evaluation of thiosemicarbazide functionalized corn bract for selective and efficient adsorption of Au(III) from aqueous solutions. J. Mol. Liq. 2018, 258, 235–243. [Google Scholar] [CrossRef]
- Bratskaya, S.Y.; Ustinov, A.Y.; Azarova, Y.A.; Pestov, A.V. Thiocarbamoyl chitosan: Synthesis, characterization and sorption of Au (III), Pt (IV), and Pd (II). Carbohydr. Polym. 2011, 85, 854–861. [Google Scholar] [CrossRef]
- Zhao, M.H.; Huang, Z.; Wang, S.X.; Zhang, L.B. Ultrahigh efficient and selective adsorption of Au(III) from water by novel Chitosan-coated MoS2 biosorbents: Performance and mechanisms. Chem. Eng. J. 2020, 401, 11. [Google Scholar] [CrossRef]
- Zhao, M.H.; Li, X.T.; Huang, Z.; Wang, S.X.; Zhang, L.B. Facile cross-link method to synthesize chitosan-based adsorbent with superior selectivity toward gold ions: Batch and column studies. Int. J. Biol. Macromol. 2021, 172, 210–222. [Google Scholar] [CrossRef]
- Dai, B.; Cao, M.; Fang, G.; Liu, B.; Dong, X.; Pan, M.; Wang, S. Schiff base-chitosan grafted multiwalled carbon nanotubes as a novel solid-phase extraction adsorbent for determination of heavy metal by ICP-MS. J. Hazard. Mater. 2012, 219–220, 103–110. [Google Scholar] [CrossRef]
- He, J.C.; Zhou, F.Q.; Mao, Y.F. Preconcentration of trace cadmium (II) and copper (II) in environmental water using a column packed with modified silica gel-chitosan prior to flame atomic absorption spectrometry determination. Anal. Lett. 2013, 46, 1430–1441. [Google Scholar] [CrossRef]
- Basuki, R.; Rusdiarso, B. Sorption-desorption profile of Au(III) onto silica modified quaternary amines (SMQA) in gold mining effluent. J. Environ. Chem. Eng. 2020, 8, 9. [Google Scholar] [CrossRef]
- Xie, Q.; Liang, G.; Lin, T.; Chen, F.; Wang, D.; Yang, B. Selective chelating precipitation of palladium metal from electroplating wastewater using chitosan and its derivative. Adsorp. Sci. Technol. 2020, 38, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Liang, H.; Lu, Y.; Xu, H.; Jiao, Y. Adsorption of gold from waste mobile phones by biochar and activated carbon in gold iodized solution. Waste Manag. 2021, 120, 530–537. [Google Scholar] [CrossRef]
- Chen, Y.; Zi, F.; Hu, X.; Yang, P.; Ma, Y.; Cheng, H.; Wang, Q.; Qin, X.; Liu, Y.; Chen, S.; et al. The use of new modified activated carbon in thiosulfate solution: A green gold recovery technology. Sep. Purif. Technol. 2020, 230, 115834. [Google Scholar] [CrossRef]
- Yu, H.; Zi, F.; Hu, X.; Nie, Y.; Chen, Y.; Cheng, H. Adsorption of gold from thiosulfate solutions with chemically modified activated carbon. Adsorp. Sci. Technol. 2018, 36, 408–428. [Google Scholar] [CrossRef]
- Bediako, J.K.; Lin, S.; Sarkar, A.K.; Zhao, Y.F.; Choi, J.W.; Song, M.H.; Wei, W.; Reddy, D.H.K.; Cho, C.W.; Yun, Y.S. Benignly-fabricated crosslinked polyethylenimine/calcium-alginate fibers as high-performance adsorbents for effective recovery of gold. J. Clean. Prod. 2020, 252, 14. [Google Scholar] [CrossRef]
- Guo, W.H.; Zhao, Z.G.; Yang, F.; Xie, M.Y.; Shao, Z.H.; Xue, L.Y.; Zhang, Y.; Lin, W.Q.; Du, C.; Zhang, Y.J. Control of the separation order of Au(III), Pd(II), and Pt(IV) achieved by site-controllable carboxyl-functionalized diethylaminoethyl celluloses. Cellulose 2020, 27, 10167–10181. [Google Scholar] [CrossRef]
- Grad, O.; Ciopec, M.; Negrea, A.; Duțeanu, N.; Vlase, G.; Negrea, P.; Dumitrescu, C.; Vlase, T.; Vodă, R. Precious metals recovery from aqueous solutions using a new adsorbent material. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I.; Martinez, M.; Miralles, N.; Poch, J.; Serarols, J. Sorption of Pb(II), Ni(II), Cu (II) and Cd (II) from aqueous solution by olive stone waste. Sep. Purif. Technol. 2006, 50, 132–140. [Google Scholar] [CrossRef]
- Tofan, L.; Bunia, I.; Paduraru, C.; Teodosiu, C. Synthesis, characterization and experimental assessment of a novel functionalized macroporous acrylic copolymer for gold separation from wastewater. Process Saf. Environ. Prot. 2017, 106, 150–162. [Google Scholar] [CrossRef]
- An, F.Q.; Li, M.; Guo, X.D.; Wang, H.Y.; Wu, R.Y.; Hu, T.P.; Gao, J.F.; Jiao, W.Z. Selective adsorption of AuCl4− on chemically modified D301 resin with containing N/S functional polymer. J. Environ. Chem. Eng. 2017, 5, 10–15. [Google Scholar] [CrossRef]
- Zhang, G.W.; Zhou, Y.; Ding, Z.; Fu, L.K.; Wang, S.X. Nanosilica-supported thiosemicarbazide-glutaraldehyde polymer for selective Au(III) removal from aqueous solution. RSC Advances 2017, 7, 55215–55223. [Google Scholar] [CrossRef] [Green Version]
- Guibal, E.; Vincent, T.; Mendoza, R.N. Synthesis and Characterization of a Thiourea Derivative of Chitosan for Platinum Recovery. J. Appl. Polym. Sci. 1999, 75, 119–134. [Google Scholar] [CrossRef]
- Arrascue, M.L.; Garcia, H.M.; Horna, O.; Guibal, E. Gold sorption on chitosan derivatives. Hydrometallurgy 2003, 71, 191–200. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Huiping, S.; Xingang, L.I.; Xiaohong, S.; Jingsheng, S.; Yanhong, W.; Zenhua, W.U. Biosorption equilibrium and kinetics of Au (III) and Cu (II) on magnetotactic bacteria. Chin. J. Chem. Eng. 2007, 15, 847–854. [Google Scholar]
- Weber, W.J.; DiGiano, F.A. Process Dynamics in Environmental Systems; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Guo, X.; Wang, J. A general kinetic model for adsorption: Theoretical analysis and modeling. J. Mol. Liq. 2019, 288, 111100. [Google Scholar] [CrossRef]
- Pethkar, A.V.; Kulkarni, K.M. Comparative studies on metal biosorption by two strains of cladosporium cladosporoides. Bioresour. Technol. 2001, 80, 211–215. [Google Scholar] [CrossRef]
- Hassan, M.M.; Mohamed, M.H.; Udoetok, I.A.; Steiger, B.G.K.; Wilson, L.D. Sequestration of sulfate anions from groundwater by biopolymer-metal composite materials. Polymers 2020, 12, 1502. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Elwakeel, K.Z. Gold (III) recovery using snythetic chelating resin with amine, thio and amine/mercaptan functionalities. Sep. Purif. Technol. 2005, 42, 111–116. [Google Scholar] [CrossRef]
- Sun, K.G.; Mao, S.J.; Zhan, W.Q.; Liu, C.; Jia, F.F.; Song, S.X. In-situ reduction of Au(S2O3)23− for efficient recovery of gold with magnetically separable shell-core structured MoS2@Fe3O4 composite. Hydrometallurgy 2020, 198, 105514. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Das, N. Recovery of precious metals through biosorption - A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Ishikawa, S.I.; Suyama, K.; Arihara, K.; Itoh, M. Uptake and recovery of gold ions from electroplating wastes using eggshell membrane. Bioresour. Technol. 2002, 81, 201–206. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Elwakeel, K.Z. Recovery of gold (III) and sliver (I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy 2007, 87, 197–206. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Udoetok, I.A.; Solgi, M.; Steiger, B.; Zhou, Z.; Wilson, L.D. Design of Sustainable Biomaterial Composite Adsorbents for Point-of-Use Removal of Lead Ions from Water. Front. Water 2022, 4, 739492. [Google Scholar] [CrossRef]
- Kong, D.; McGilp, L.; Klyashtorin, A.; Wilson, I.; Wilson, L.D. Pilot Test of the Permeable Reactive Barrier for Removing Uranium from the Flooded Gunnar Pit. J. Geo. Sci. Environ. Prot. 2020, 8, 155–176. [Google Scholar] [CrossRef]
- Hsu, E.; Barmak, K.; Westa, A.C.; Park, A.H.A. Advancements in the treatment and processing of electronic waste with sustainability: A review of metal extraction and recovery technologies. Green Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]








| Adsorbent | Characterization Methods | Metal Ions | Uptake Qm (mg/g) | Conditions | Literature Refs. | |||
|---|---|---|---|---|---|---|---|---|
| pH | T (K) | Time (h) | Dosage (g/L) | |||||
| GCC beads | SEM, XPS, TGA, FTIR | Au(III) | 600 | 2 | 283, 298 | 24 | 0.2–1.5 | [22] |
| GCC Beads | XPS, TGA, FTIR | Au(III), Pd(II) | 240 (Au), 120 (Pd) | 1–10 | 298 | 0.5 to 24 | 1–15 | [23] |
| GCC beads | TGA, SEM, EDX, | Au(III) | 400 | 0.2–4 | 298 | 24 | 1 | [24] |
| Adsorbent | Characterization Methods | Metal Ions | Uptake Qm (mg/g) | Conditions | Literature Refs. | |||
|---|---|---|---|---|---|---|---|---|
| pH | T (K) | Time (h) | Dosage (g/L) | |||||
| IMC | XPS, AAS, 1H NMR, & XRD | Au(III), Pt(IV), Pd(II) | 158 46 68 | 2–9 | 298 | 18 | 1.00 | [27] |
| DAVF-CS | XPS, 13C NMR, FTIR, TGA, and elemental analysis | Au(III), Pd(II) | 322 (Au), 207 (Pd) | 1–6 | 298 | 24 | 0.2 | [30] |
| NCMC | FTIR | Au(III) | 30 | 2–12 | 298 | 30 | 2 | [31] |
| Au(III), Pd(II) | 69.9 (Au), 58.6 (Pd) | 2, 3 | [28] | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Foley, S.R.; Wilson, L.D. An Overview of Modified Chitosan Adsorbents for the Removal of Precious Metals Species from Aqueous Media. Molecules 2022, 27, 978. https://doi.org/10.3390/molecules27030978
Kong D, Foley SR, Wilson LD. An Overview of Modified Chitosan Adsorbents for the Removal of Precious Metals Species from Aqueous Media. Molecules. 2022; 27(3):978. https://doi.org/10.3390/molecules27030978
Chicago/Turabian StyleKong, Dexu, Stephen R. Foley, and Lee D. Wilson. 2022. "An Overview of Modified Chitosan Adsorbents for the Removal of Precious Metals Species from Aqueous Media" Molecules 27, no. 3: 978. https://doi.org/10.3390/molecules27030978