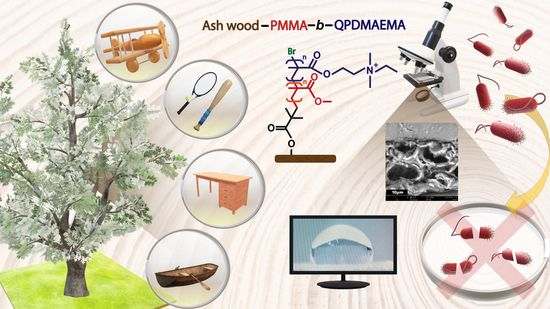
A New Protocol for Ash Wood Modification: Synthesis of Hydrophobic and Antibacterial Brushes from the Wood Surface
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Concept of a “Grafting from” Approach for the Modification of Ash Wood Cubes
2.2. Preparation of Wood–Polymer Composites with Dual Characteristics
2.3. The Unique Properties of Wood Grafted with Polymers
3. Experimental
3.1. Chemicals
3.2. Analysis
3.2.1. Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR)
3.2.2. Gel Permeation Chromatography (GPC)
3.2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
3.2.4. Scanning Electron Microscopy (SEM)
3.2.5. Atomic Force Microscopy (AFM)
3.2.6. Water Contact Angle Measurements
3.2.7. Antibacterial Activity Assays
3.2.8. Antibacterial activity of modified wood
3.2.9. Minimum Inhibitory/Minimum Bactericidal Concentration (MIC/MBC)
3.2.10. Statistical Analysis
3.3. Immobilization of BriBBr on Ash Wood Surface (Wood-Br)
3.4. General Procedure for Ag0 SI-ARGET ATRP of MMA from a Wood Surfaces
3.5. General Procedure for SI-ARGET ATRP of DMAEMA from a Wood Surfaces
3.6. Measurements of Water and NaCl Solution Absorption
3.7. Quaternization of PDMAEMA Grafted from the Wood Surface
3.8. Quaternization of PDMAEMA from Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Burgert, I.; Keplinger, T. Plant micro- and nanomechanics: Experimental techniques for plant cell-wall analysis. J. Exp. Bot. 2013, 64, 4635–4649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgert, I.; Cabane, E.; Zollfrank, C.; Berglund, L. Bio-inspired functional wood-based materials—Hybrids and replicates. Int. Mater. Rev. 2015, 60, 431–450. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Chu, F. Wood-derived functional polymeric materials. Adv. Mater. 2021, 33, 2001135. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.Y.; Chui, Y.H.; Wan, H. Effect of impregnation and in-situ polymerization of methacrylates on hardness of sugar maple wood. J. Appl. Polym. Sci. 2006, 99, 1674–1683. [Google Scholar] [CrossRef]
- Nzokou, P.; Kamdem, D.P.; Temiz, A. Effect of accelerated weathering on discoloration and roughness of finished ash wood surfaces in comparison with red oak and hard maple. Prog. Org. Coat. 2011, 71, 350–354. [Google Scholar] [CrossRef]
- Zhong, Z.W.; Hiziroglu, S.; Chan, C.T.M. Measurement of the surface roughness of wood based materials used in furniture manufacture. Measurement 2013, 46, 1482–1487. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Dong, X.; Fu, Y.; Liu, Y. Comparison of decay resistance of wood and wood-polymer composite prepared by in-situ polymerization of monomers. Int. Biodeterior. Biodegrad. 2013, 84, 401–406. [Google Scholar] [CrossRef]
- Kong, L.; Guan, H.; Wang, X. In situ polymerization of furfuryl alcohol with ammonium dihydrogen phosphate in poplar wood for Improved dimensional stability and flame retardancy. ACS Sustain. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Gérardin, P. New alternatives for wood preservation based on thermal and chemical modification of wood—A review. Ann. For. Sci. 2016, 73, 559–570. [Google Scholar] [CrossRef] [Green Version]
- Van Gorkum, R.; Bouwman, E. The oxidative drying of alkyd paint catalysed by metal complexes. Coord. Chem. Rev. 2005, 249, 1709–1728. [Google Scholar] [CrossRef]
- Custódio, J.E.P.; Eusébio, M.I. Waterborne acrylic varnishes durability on wood surfaces for exterior exposure. Prog. Org. Coat. 2006, 56, 59–67. [Google Scholar] [CrossRef]
- Li, Y.-F.; Liu, Y.-X.; Wang, X.-M.; Wu, Q.-L.; Yu, H.-P.; Li, J. Wood–polymer composites prepared by the in situ polymerization of monomers within wood. J. Appl. Polym. Sci. 2011, 119, 3207–3216. [Google Scholar] [CrossRef]
- Cabane, E.; Keplinger, T.; Merk, V.; Hass, P.; Burgert, I. Renewable and functional wood materials by grafting polymerization within cell walls. ChemSusChem 2014, 7, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Sèbe, G.; Brook, M.A. Hydrophobization of wood surfaces: Covalent grafting of silicone polymers. Wood Sci. Technol. 2001, 35, 269–282. [Google Scholar] [CrossRef]
- Hakkou, M.; Pétrissans, M.; Zoulalian, A.; Gérardin, P. Investigation of wood wettability changes during heat treatment on the basis of chemical analysis. Polym. Degrad. 2005, 89, 1–5. [Google Scholar] [CrossRef]
- Fu, Y.; Li, G.; Yu, H.; Liu, Y. Hydrophobic modification of wood via surface-initiated ARGET ATRP of MMA. Appl. Surf. Sci. 2012, 258, 2529–2533. [Google Scholar] [CrossRef]
- Ermeydan, M.A.; Cabane, E.; Gierlinger, N.; Koetz, J.; Burgert, I. Improvement of wood material properties via in situ polymerization of styrene into tosylated cell walls. RSC Adv. 2014, 4, 12981–12988. [Google Scholar] [CrossRef] [Green Version]
- Zaborniak, I.; Macior, A.; Chmielarz, P.; Smenda, J.; Wolski, K. Hydrophobic modification of fir wood surface via low ppm ATRP strategy. Polymer 2021, 228, 123942. [Google Scholar] [CrossRef]
- Deka, B.K.; Mandal, M.; Maji, T.K. Effect of nanoparticles on flammability, UV resistance, biodegradability, and chemical resistance of wood polymer nanocomposite. Ind. Eng. Chem. Res. 2012, 51, 11881–11891. [Google Scholar] [CrossRef]
- Ermeydan, M.A.; Cabane, E.; Hass, P.; Koetz, J.; Burgert, I. Fully biodegradable modification of wood for improvement of dimensional stability and water absorption properties by poly(ε-caprolactone) grafting into the cell walls. Green Chem. 2014, 16, 3313–3321. [Google Scholar] [CrossRef] [Green Version]
- Vedrtnam, A.; Kumar, S.; Chaturvedi, S. Experimental study on mechanical behavior, biodegradability, and resistance to natural weathering and ultraviolet radiation of wood-plastic composites. Compos. Part B Eng. 2019, 176, 107282. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Jia, X.; Li, Y.; Wang, S.; Song, H. Wood-based solar interface evaporation device with self-desalting and high antibacterial activity for efficient solar steam generation. ACS Appl. Mater. Interfaces 2020, 12, 47029–47037. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Shen, X.; Fu, F.; Yang, S.; Li, G.; Chu, F. Improving physical properties of wood–polymer composites by building stable interface structure between swelled cell walls and hydrophobic polymer. Wood Sci. Technol. 2021, 55, 1401–1417. [Google Scholar] [CrossRef]
- Devi, R.R.; Maji, T.K. Effect of nanofillers on flame retardancy, chemical resistance, antibacterial properties and biodegradation of wood/styrene acrylonitrile co-polymer composites. Wood Sci. Technol. 2013, 47, 1135–1152. [Google Scholar] [CrossRef]
- Devi, R.R.; Gogoi, K.; Konwar, B.K.; Maji, T.K. Synergistic effect of nanoTiO2 and nanoclay on mechanical, flame retardancy, UV stability, and antibacterial properties of wood polymer composites. Polym. Bull. 2013, 70, 1397–1413. [Google Scholar] [CrossRef]
- Gerullis, S.; Pfuch, A.; Spange, S.; Kettner, F.; Plaschkies, K.; Küzün, B.; Kosmachev, P.V.; Volokitin, G.G.; Grünler, B. Thin antimicrobial silver, copper or zinc containing SiOx films on wood polymer composites (WPC) applied by atmospheric pressure plasma chemical vapour deposition (APCVD) and sol–gel technology. Eur. J. Wood Wood Prod. 2018, 76, 229–241. [Google Scholar] [CrossRef]
- Basu, A.; Heitz, K.; Strømme, M.; Welch, K.; Ferraz, N. Ion-crosslinked wood-derived nanocellulose hydrogels with tunable antibacterial properties: Candidate materials for advanced wound care applications. Carbohydr. Polym. 2018, 181, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Modification of wood-based materials by atom transfer radical polymerization methods. Eur. Polym. J. 2019, 120, 109253. [Google Scholar] [CrossRef]
- Cheng, L.; Ren, S.; Lu, X. Application of eco-friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polymers 2020, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Siegwart, D.J.; Oh, J.K.; Matyjaszewski, K. ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012, 37, 18–37. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.-N.; Xu, L.; Liu, Q.-D.; Zhang, W.; Jia, R.; Liu, C.-Z.; Wang, S.-S.; Wang, L.-P.; Li, G. Surface-induced ARGET ATRP for silicon nanoparticles with fluorescent polymer brushes. Polymers 2019, 11, 1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-deactivation radical polymerization (Controlled/living radical polymerization): From discovery to materials design and applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Nicolaÿ, R.; Kwak, Y.; Matyjaszewski, K. A green route to well-defined high-molecular-weight (co)polymers using ARGET ATRP with alkyl pseudohalides and copper catalysis. Angew. Chem. Int. Ed. 2010, 49, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Corbin, D.A.; Miyake, G.M. Photoinduced organocatalyzed atom transfer radical polymerization (O-ATRP): Precision polymer synthesis using organic photoredox catalysis. Chem. Rev. 2022, 122, 1830–1874. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, G.; Fu, L.; Jafari, H.; Kapil, K.; Matyjaszewski, K. Making ATRP more practical: Oxygen tolerance. Acc. Chem. Res. 2021, 54, 1779–1790. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization: From mechanisms to applications. Isr. J. Chem. 2012, 52, 206–220. [Google Scholar] [CrossRef]
- Yin, R.; Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Tuning dispersity of linear polymers and polymeric brushes grown from nanoparticles by atom transfer radical polymerization. Polym. Chem. 2021, 12, 6071–6082. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Jakubowski, W.; Min, K.; Tang, W.; Huang, J.; Braunecker, W.A.; Tsarevsky, N.V. Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 15309–15314. [Google Scholar] [CrossRef] [Green Version]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Yu, H.P.; Fu, Y.C.; Li, G.; Liu, Y.X. Antimicrobial surfaces of quaternized poly (2-dimethyl amino)ethyl methacrylate grafted on wood via ARGET ATRP. Holzforschung 2013, 67, 455–461. [Google Scholar] [CrossRef]
- Cabane, E.; Keplinger, T.; Kunniger, T.; Merk, V.; Burgert, I. Functional lignocellulosic materials prepared by ATRP from a wood scaffold. Sci. Rep. 2016, 6, 31287. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wang, K.; Hu, D. High retreatability and dimensional stability of polymer grafted waterlogged archaeological wood achieved by ARGET ATRP. Sci. Rep. 2019, 9, 9879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Wang, K.; Hu, D. Surface-initiated ARGET ATRP for maintaining the dimension of waterlogged archaeological wood (Pinus massoniana): Polymer distribution behaviors and anti-shrinkage mechanism. Wood Sci. Technol. 2021, in press. [CrossRef]
- Vidiella del Blanco, M.; Gomez, V.; Fleckenstein, P.; Keplinger, T.; Cabane, E. Grafting of amphiphilic block copolymers on lignocellulosic materials via SI-AGET-ATRP. J. Polym. Sci. A Polym. Chem. 2019, 57, 885–897. [Google Scholar] [CrossRef]
- Williams, V.A.; Matyjaszewski, K. Expanding the ATRP toolbox: Methacrylate polymerization with an elemental silver reducing agent. Macromolecules 2015, 48, 6457–6464. [Google Scholar] [CrossRef]
- Williams, V.A.; Ribelli, T.G.; Chmielarz, P.; Park, S.; Matyjaszewski, K. A silver bullet: Elemental silver as an efficient reducing agent for atom transfer radical polymerization of acrylates. J. Am. Chem. Soc. 2015, 137, 1428–1431. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of inositol-based star polymers through low ppm ATRP methods. Polym. Adv. Technol. 2017, 28, 1804–1812. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Flejszar, M.; Surmacz, K.; Ostatek, R. Preparation of hydrophobic tannins-inspired polymer materials via low-ppm ATRP methods. Polym. Adv. Technol. 2020, 31, 913–921. [Google Scholar] [CrossRef]
- Dong, H.; Matyjaszewski, K. ARGET ATRP of 2-(dimethylamino)ethyl methacrylate as an intrinsic reducing agent. Macromolecules 2008, 41, 6868–6870. [Google Scholar] [CrossRef]
- Teper, P.; Chojniak-Gronek, J.; Hercog, A.; Oleszko-Torbus, N.; Płaza, G.; Kubacki, J.; Balin, K.; Kowalczuk, A.; Mendrek, B. Nanolayers of poly(NN′-dimethylaminoethyl methacrylate) with a star topology and their antibacterial activity. Polymers 2020, 12, 230. [Google Scholar] [CrossRef] [Green Version]
- Mosnáček, J.; Ilčíková, M. Photochemically mediated atom transfer radical polymerization of methyl methacrylate using ppm amounts of catalyst. Macromolecules 2012, 45, 5859–5865. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, D.; Shi, Y.; Yang, W.; Fu, Z. Polymerization mechanism of MMA in the presence of 1,1-diphenylethylene. Macromol. Chem. Phys. 2013, 214, 1688–1698. [Google Scholar] [CrossRef]
- Couthouis, J.; Keul, H.; Möller, M. MALDI-TOF analysis of halogen telechelic poly(methyl methacrylate)s and poly(methyl acrylate)s prepared by atom transfer radical polymerization (ATRP) or single electron transfer-living radical polymerization (SET-LRP). Macromol. Chem. Phys. 2015, 216, 1791–1800. [Google Scholar] [CrossRef]
- Wesley, R.D.; Dreiss, C.A.; Cosgrove, T.; Armes, S.P.; Thompson, L.; Baines, F.L.; Billingham, N.C. Structure of a hydrophilic−hydrophobic block copolymer and its interactions with salt and an anionic surfactant. Langmuir 2005, 21, 4856–4861. [Google Scholar] [CrossRef]
- Xiao, G.; Hu, Z.; Zeng, G.; Wang, Y.; Huang, Y.; Hong, X.; Xia, B.; Zhang, G. Effect of hydrophilic chain length on the aqueous solution behavior of block amphiphilic copolymers PMMA-b-PDMAEMA. J. Appl. Polym. Sci. 2012, 124, 202–208. [Google Scholar] [CrossRef]
- Zhu, H.; Fang, Z.; Wang, Z.; Dai, J.; Yao, Y.; Shen, F.; Preston, C.; Wu, W.; Peng, P.; Jang, N.; et al. Extreme light management in mesoporous wood cellulose paper for optoelectronics. ACS Nano 2016, 10, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef] [PubMed]
- Meier, H.E.M.; Kjellström, E.; Graham, L.P. Estimating uncertainties of projected Baltic Sea salinity in the late 21st century. Geophys. Res. Lett. 2006, 33, L15705. [Google Scholar] [CrossRef]
- Munir, M.T.; Pailhories, H.; Eveillard, M.; Irle, M.; Aviat, F.; Dubreil, L.; Federighi, M.; Belloncle, C. Testing the antimicrobial characteristics of wood materials: A review of methods. Antibiotics 2020, 9, 225. [Google Scholar] [CrossRef]
- Koufakis, E.; Manouras, T.; Anastasiadis, S.H.; Vamvakaki, M. Film properties and antimicrobial efficacy of quaternized PDMAEMA brushes: Short vs. long alkyl chain length. Langmuir 2020, 36, 3482–3493. [Google Scholar] [CrossRef]
- Drauch, V.; Ibesich, C.; Vogl, C.; Hess, M.; Hess, C. In-vitro testing of bacteriostatic and bactericidal efficacy of commercial disinfectants against Salmonella Infantis reveals substantial differences between products and bacterial strains. Int. J. Food Microbiol. 2020, 328, 108660. [Google Scholar] [CrossRef] [PubMed]
- Drugbank Online. Available online: https://go.drugbank.com/drugs/DB00713 (accessed on 2 December 2021).
- Xu, L.Q.; Li, N.N.; Chen, J.C.; Fu, G.D.; Kang, E.-T. Quaternized poly(2-(dimethylamino)ethyl methacrylate)-grafted agarose copolymers for multipurpose antibacterial applications. RSC Adv. 2015, 5, 61742–61751. [Google Scholar] [CrossRef]
- Kaur, A.; Ribelli, T.G.; Schröder, K.; Matyjaszewski, K.; Pintauer, T. Properties and ATRP activity of copper complexes with substituted tris(2-pyridylmethyl)amine-based ligands. Inorg. Chem. 2015, 54, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Krys, P.; Wang, Z.; Wang, Y.; Matyjaszewski, K. Synthesis of well-defined polymer brushes from silicon wafers via surface-initiated seATRP. Macromol. Chem. Phys. 2017, 218, 1700106. [Google Scholar] [CrossRef]
- Flejszar, M.; Chmielarz, P.; Wolski, K.; Grześ, G.; Zapotoczny, S. Polymer brushes via surface-initiated electrochemically mediated ATRP: Role of a sacrificial initiator in polymerization of acrylates on silicon substrates. Materials 2020, 13, 3559. [Google Scholar] [CrossRef]
- Gao, L.; Gan, W.; Xiao, S.; Zhan, X.; Li, J. A robust superhydrophobic antibacterial Ag–TiO2 composite film immobilized on wood substrate for photodegradation of phenol under visible-light illumination. Ceram. Int. 2016, 42, 2170–2179. [Google Scholar] [CrossRef]
- Xue, C.-H.; Chen, J.; Yin, W.; Jia, S.-T.; Ma, J.-Z. Superhydrophobic conductive textiles with antibacterial property by coating fibers with silver nanoparticles. Appl. Surf. Sci. 2012, 258, 2468–2472. [Google Scholar] [CrossRef]
- Bocian, A.; Ciszkowicz, E.; Hus, K.K.; Buczkowicz, J.; Lecka-Szlachta, K.; Pietrowska, M.; Petrilla, V.; Petrillova, M.; Legáth, L.; Legáth, J. Antimicrobial activity of protein fraction from naja ashei venom against staphylococcus epidermidis. Molecules 2020, 25, 293. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Walther, A.; André, X.; Lanzendörfer, M.G.; Müller, A.H.E. Synthesis of highly branched cationic polyelectrolytes via self-condensing atom transfer radical copolymerization with 2-(diethylamino)ethyl methacrylate. Macromolecules 2004, 37, 2054–2066. [Google Scholar] [CrossRef]












| Entry | [MMA]0/[EBiB]0/[CuIIBr2/TPMA]0 | [CuIIBr2]0, (ppm by wt) | Conv 2 (%) | kpapp 3 (h−1) | DPn,theo 2 (per chain) | Mn,theo4 (×10−3) | Mn,app5 (×10−3) | Mw/Mn5 |
|---|---|---|---|---|---|---|---|---|
| 1 | 600/1/0.180 | 273 | 67 | 0.124 | 400 | 40.2 | 21.6 | 1.44 |
| 2 | 600/1/0.120 | 182 | 60 | 0.088 0.087 6 | 362 | 36.5 | 22.2 | 1.43 |
| 3 | 600/1/0.060 | 90 | 62 | 0.101 0.104 6 | 370 | 37.2 | 20.6 | 1.33 |
| 4 | 600/1/0.048 | 72 | 71 | 0.120 | 426 | 42.8 | 17.9 | 1.31 |
| 5 | 600/1/0.006 | 9 | 69 | 0.110 0.108 6 | 415 | 41.7 | 17.8 | 2.08 |
| Entry | [DMAEMA]0/ [EBiB]0/[CuIIBr2/TPMA]0 | [CuIIBr2]0, (ppm by wt) | Reducing Agent | Conv 2 (%) | kpapp 3 (h−1) | DPn,theo 2 (per chain) | Mn,theo4 (×10−3) | Mn,app5 (×10−3) | Mw/Mn5 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 600/1/0.048 | 45 | Ag0 | 37 | 0.064 | 225 | 35.5 | 31.4 | 1.64 |
| 2 | 600/1/0.048 | 45 | - | 18 | 0.025 | 107 | 17.1 | 20.8 | 1.42 |
| 3 | 600/1/0.060 | 57 | - | 23 | 0.033 | 136 | 21.6 | 20.7 | 1.41 |
| Entry | Monomer | [CuIIBr2]0, (ppm by wt) | Reducing Agent | Conv 2 (%) | kpapp 3 (h−1) | DPn,theo 2 (per chain) | Mn,theo4 (×10−3) | Mn,app5 (×10−3) | Mw/Mn5 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MMA | 72 | Ag0 | 71 | 0.120 | 426 | 42.8 | 17.9 | 1.31 |
| 2 6 | DMAEMA | 45 | - | 20 | 0.027 | 120 | 19.1 | 20.2 | 1.41 |
| Samples | Number of Disinfection Cycles | Survival Fraction | Log Reduction (CFU/mL) | % Reduction |
|---|---|---|---|---|
| Gram-positive Staphylococcus aureus | ||||
| Wood-PMMA-b-QPDMAEMA-Br | - * | 0.008 ± 0.0005 | 1.9 | 99.17 ± 0.08 |
| Wood-QPDMAEMA-Br | - * | 0.00003 ± 0.0003 | 3.95 | 99.997 ± 0.13 |
| 1 | 0.0046 ± 0.0005 | 2.32 | 99.54 ± 0.15 | |
| 2 | 0.027 ± 0.001 | 1.4 | 97.28 ± 0.83 | |
| Gram-negative Escherichia coli | ||||
| Wood-PMMA-b-QPDMAEMA-Br | - * | 0.18 ± 0.02 | 0.73 | 81.88 ± 1.68 |
| Wood-QPDMAEMA-Br | - * | 0.07 ± 0.002 | 1.12 | 92.58 ± 0.54 |
| 1 | 0.09 ± 0.001 | 1.04 | 90.97 ± 0.82 | |
| 2 | 0.23 ± 0.001 | 0.64 | 77.46 ± 0.54 | |
| Antibacterial Agent | Escherichia coli | Staphylococcus aureus |
|---|---|---|
| MIC/MBC (µg/mL) | ||
| QPDMAEMA | 52.97/52.97 | 6.62/13.24 |
| MIC/MBC (µg/mL) | ||
| Tetracycline | 0.5/0.5 | 0.12/0.12 |
| Gentamicin | 1.9/1.9 | 0.9/0.9 |
| Oxacillin | 125/125 | 0.12/> 0.49 |
| Rifampicin | 3.91/3.91 | < 0.01/> 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macior, A.; Zaborniak, I.; Chmielarz, P.; Smenda, J.; Wolski, K.; Ciszkowicz, E.; Lecka-Szlachta, K. A New Protocol for Ash Wood Modification: Synthesis of Hydrophobic and Antibacterial Brushes from the Wood Surface. Molecules 2022, 27, 890. https://doi.org/10.3390/molecules27030890
Macior A, Zaborniak I, Chmielarz P, Smenda J, Wolski K, Ciszkowicz E, Lecka-Szlachta K. A New Protocol for Ash Wood Modification: Synthesis of Hydrophobic and Antibacterial Brushes from the Wood Surface. Molecules. 2022; 27(3):890. https://doi.org/10.3390/molecules27030890
Chicago/Turabian StyleMacior, Angelika, Izabela Zaborniak, Paweł Chmielarz, Joanna Smenda, Karol Wolski, Ewa Ciszkowicz, and Katarzyna Lecka-Szlachta. 2022. "A New Protocol for Ash Wood Modification: Synthesis of Hydrophobic and Antibacterial Brushes from the Wood Surface" Molecules 27, no. 3: 890. https://doi.org/10.3390/molecules27030890