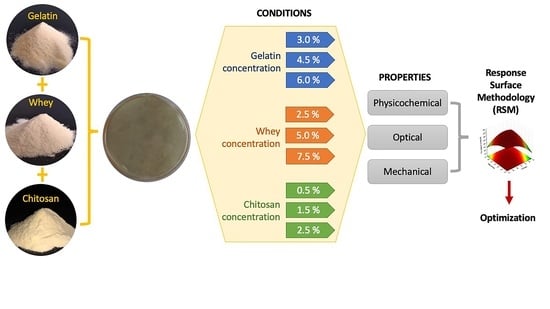
Optimization of the Physical, Optical and Mechanical Properties of Composite Edible Films of Gelatin, Whey Protein and Chitosan
Abstract
:1. Introduction
2. Results and Discussions
2.1. Adjustment of the Surface Response Methodology Model
2.2. Determination of the Second-Order Polynomial Mathematical Models
2.3. Effect of the Independent Variables over the Viscosity of the Edible Film-Forming Solution Composed of G–W–C
2.4. Effect of the Independent Variables over the Physical Properties of the Composite Edible Films of G–W–C
2.5. Effect of the Independent Variables over the Optical Properties of the Edible Films Composed of G–W–C
2.6. Effect of the Independent Variables over the Mechanical Properties of the Edible Films Composed of G–W–C
2.7. Optimization and Validation of the Box–Behnken Design
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Film-Forming Solution
3.3. Viscosity of the Film-Forming Solutions
3.4. Film Preparation
3.5. Thickness
3.6. Moisture Content
3.7. Water Absorption Capacity (WAC)
3.8. Water Solubility
3.9. Water Vapor Permeability (WVP)
3.10. Color
3.11. Light Transmission and Transparency of the Films
3.12. Mechanical Properties
3.13. Experimental Design
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rasheed, M.; Parveez, B. Coating on packaging products to enhance shelf life. In Biopolymers and Biocomposites from Agro-Waste for Packaging Applications; Saba, N., Jawaid, M., Thariq, M., Eds.; Series in Composites Science and, Engineering; Woodhead Publishing: Cambridge, UK, 2021; pp. 1–33. [Google Scholar] [CrossRef]
- Moeini, A.; Germann, N.; Malinconico, M.; Santagata, G. Formulation of secondary compounds as additives of biopolymer-based food packaging: A review. Trends Food Sci. Technol. 2021, 114, 342–354. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-based packaging: Materials, modifications, industrial applications and sustainability: Review. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Garrido, T.; Peñalba, M.; de la Caba, K.; Guerrero, P. A more efficient process to develop protein films derived from agro-industrial by-products. Food Hydrocoll. 2017, 86, 11–17. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Daniloski, D.; Petkoska, A.T.; Lee, N.A.; Bekhit, A.E.-D.; Carne, A.; Vaskoska, R.; Vasiljevic, T. Active edible packaging based on milk proteins: A route to carry and deliver nutraceuticals. Trends Food Sci. Technol. 2021, 111, 688–705. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Kurek, M.; Bornaz, S.; Debeaufort, F. Barrier, structural and mechanical properties of bovine gelatin-chitosan blend films related to biopolymer interactions. J. Sci. Food Agric. 2014, 94, 2409–2419. [Google Scholar] [CrossRef]
- Żelaziński, T. Properties of Biocomposites from Rapeseed Meal, Fruit Pomace and Microcrystalline Cellulose Made by Press Pressing: Mechanical and Physicochemical Characteristics. Materials. 2021, 14, 890. [Google Scholar] [CrossRef]
- Żelaziński, T.; Ekielski, A.; Siwek, A.; Dardziński, L. Characterisation of corn extrudates with the addition of brewers’ spent grain as a raw material for the production of functional batters. Acta Sci. Pol. Technol. Aliment. 2017, 16, 247–254. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- García-Argueta, I.; Dublán-García, O.; Quintero-Salazar, B.; Dominguez-Lopez, A.; Gómez-Oliván, L.M.; Salem, A. Effect of lactic acid bacteria on the textural properties of an edible film based on whey, inulin and gelatin. Afr. J. Biotechnol. 2013, 12, 2659–2669. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Montero, P. Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin–chitosan films. Food Hydrocoll. 2011, 25, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Morachis-Valdez, A.G.; Santillán-Álvarez, Á.; Gómez-Oliván, L.M.; García-Argueta, I.; Islas-Flores, H.; Dublán-García, O. Effects of peppermint extract and chitosan-based edible coating on storage quality of common Carp (Cyprinus carpio) fillets. Polymers 2021, 19, 3243. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Ahmed, J. Rheological properties of gelatin and advances in measurement. In Advances in Food Rheology and Its Applications; Ahmed, J., Ptaszek, P., Basu, S., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2017; pp. 377–404. [Google Scholar] [CrossRef]
- de Lacey, A.L.; López-Caballero, M.; Gómez-Estaca, J.; Gómez-Guillén, M.; Montero, P. Functionality of Lactobacillus acidophilus and Bifidobacterium bifidum incorporated to edible coatings and films. Innov. Food Sci. Emerg. Technol. 2012, 16, 277–282. [Google Scholar] [CrossRef]
- Neves, A.C.V., Jr.; Coneglian, R.C.C.; Soares, A.G.; Freitas, D.G.C.; Fonseca, M.J.O.; Barreira, F.R. Physical and sensory characterization of edible coatings applied to minimally processed persimmon. Acta Hortic. 2012, 934, 537–542. [Google Scholar] [CrossRef]
- Jooyandeh, H. Whey protein films and coatings: A review. Pak. J. Nutr. 2011, 10, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Janjarasskul, T.; Tananuwong, K. Role of whey proteins in food packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Water vapor permeability and solubility of films from hydrolyzed whey protein. J. Food Sci. 2000, 65, 700–703. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Sharma, R. Whey proteins. In Functional Foods; Deeth, H.C., Bansal, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 637–663. [Google Scholar] [CrossRef]
- Schmid, M.; Müller, K. Whey protein-based packaging films and coatings. In Functional Foods; Deeth, H.C., Bansal, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 407–437. [Google Scholar] [CrossRef]
- Di Pierro, P.; Mariniello, L.; Giosafatto, V.L.; Esposito, M.; Sabbah, M.; Porta, R. Dairy whey protein-based edible films and coatings for food preservation. In Handbook of Food Bioengineering, Food Packaging and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 439–456. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Jridi, M.; Hajji, S.; Ben Ayed, H.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin–chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Akyüz, L.; Kaya, M.; Ilk, S.; Cakmak, Y.S.; Salaberria, A.M.; Labidi, J.; Yılmaz, B.A.; Sargin, I. Effect of different animal fat and plant oil additives on physicochemical, mechanical, antimicrobial and antioxidant properties of chitosan films. Int. J. Biol. Macromol. 2018, 111, 475–484. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Rocha, C.M.; Avides, M.C.; Quintas, M.A.; Vicente, A.A. Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight. J. Food Eng. 2011, 106, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Debeaufort, F.; Quezada-Gallo, J.-A.; Voilley, A. Edible films and coatings: Tomorrow’s packagings: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 299–313. [Google Scholar] [CrossRef]
- Calva-Estrada, S.; Jimenez-Fernández, M.; Lugo-Cervantes, E. Protein-based films: Advances in the development of biomaterials applicable to food packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Javidi, Z.; Rezaei, M. Efficient gas barrier properties of multi-layer films based on poly(lactic acid) and fish gelatin. Int. J. Biol. Macromol. 2016, 92, 1205–1214. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Auty, M.A.; Kerry, J.P. Physical assessment of composite biodegradable films manufactured using whey protein isolate, gelatin and sodium alginate. J. Food Eng. 2010, 96, 199–207. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.; Pinotti, A. Composite and bi-layer films based on gelatin and chitosan. J. Food Eng. 2009, 90, 531–539. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Sobral, P.J. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Farsanipour, A.; Khodanazary, A.; Hosseini, S.M. Effect of chitosan-whey protein isolated coatings incorporated with tarragon Artemisia dracunculus essential oil on the quality of of Nemipterus japonicus fillets at refrigerated condition. Int. J. Biol. Macromol. 2020, 155, 766–771. [Google Scholar] [CrossRef]
- Ferreira, C.O.; Nunes, C.A.; Delgadillo, I.; Lopes-Da-Silva, J. Characterization of chitosan–whey protein films at acid pH. Food Res. Int. 2009, 42, 807–813. [Google Scholar] [CrossRef]
- Sharma, L.; Singh, C. Sesame protein based edible films: Development and characterization. Food Hydrocoll. 2016, 61, 139–147. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Affes, S.; Boufi, S.; Nasri, M. Optimization of the formulation of chitosan edible coatings supplemented with carotenoproteins and their use for extending strawberries postharvest life. Food Hydrocoll. 2018, 83, 375–392. [Google Scholar] [CrossRef]
- Tomadoni, B.; Ponce, A.; Pereda, M.; Ansorena, M. Vanillin as a natural cross-linking agent in chitosan-based films: Optimizing formulation by response surface methodology. Polym. Test. 2019, 78, 105935. [Google Scholar] [CrossRef]
- Bekale, L.; Agudelo, D.; Tajmir-Riahi, H. Effect of polymer molecular weight on chitosan–protein interaction. Colloids Surfaces B Biointerfaces 2015, 125, 309–317. [Google Scholar] [CrossRef]
- Akdoğan, Y.; Reichenwallner, J.; Hinderberger, D. Evidence for water-tuned structural differences in proteins: An approach emphasizing variations in local hydrophilicity. PLoS ONE 2012, 7, e45681. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Luo, X.; Betz, J.; Buckhout-White, S.; Bekdash, O.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. In situ quantitative visualization and characterization of chitosan electrodeposition with paired sidewall electrodes. Soft Matter 2010, 6, 3177–3183. [Google Scholar] [CrossRef]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Domard, A. pH and c.d. measurements on a fully deacetylated chitosan: Application to CuII-polymer interactions. Int. J. Biol. Macromol. 1987, 9, 98–104. [Google Scholar] [CrossRef]
- Sperling, L.H. Interpenetrating polymer networks: An overview. Adv. Chem. 1994, 239, 3–38. [Google Scholar] [CrossRef]
- Panteli, P.A.; Patrickios, C.S. Multiply interpenetrating polymer networks: Preparation, mechanical properties, and applications. Review. Gels 2019, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.; Mohammadifar, M.A.; Rouhi, M.; Kariminejad, M.; Mortazavian, A.M.; Sadeghi, E.; Hasanvand, S. Physico-mechanical and structural properties of eggshell membrane gelatin-chitosan blend edible films. Int. J. Biol. Macromol. 2018, 107, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Pranoto, Y.; Rakshit, S.; Salokhe, V. Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT-Food Sci. Technol. 2005, 38, 859–865. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Rosa, G.B.; Ferreira, A.L.A.; da Rosa, C.G.; Beling, P.C.; Xavier, L.O.; Hansen, C.M.; Ferrareze, J.P.; Nunes, M.R.; Barreto, P.L.M.; et al. Bioactive food packaging based on starch, citric pectin and functionalized with Acca sellowiana waste by-product: Characterization and application in the postharvest conservation of apple. Int. J. Biol. Macromol. 2020, 147, 295–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.H. Plasticization of pea starch films with monosaccharides and polyols. J. Food Sci. 2006, 71, E253–E261. [Google Scholar] [CrossRef]
- Mesa-Ospina, N.; Escobar-Sierra, D.M. Evaluación de recubrimientos de quitosano sobre cuerpos porosos de hidroxiapatita. Sci. Tech. XXIV 2019, 24, 161–172. [Google Scholar]
- Rao, M.; Kanatt, S.; Chawla, S.; Sharma, A. Chitosan and guar gum composite films: Preparation, physical, mechanical and antimicrobial properties. Carbohydr. Polym. 2010, 82, 1243–1247. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.; Chockchaisawasdee, S.; Golding, J.; Scarlett, C.J.; Stathopoulos, C.E. Mechanical and physical properties of pea starch edible films in the presence of glycerol. J. Food Process. Preserv. 2016, 40, 1339–1351. [Google Scholar] [CrossRef]
- Bajić, M.; Oberlintner, A.; Kõrge, K.; Likozar, B.; Novak, U. Formulation of active food packaging by design: Linking composition of the film-forming solution to properties of the chitosan-based film by response surface methodology (RSM) modelling. Int. J. Biol. Macromol. 2020, 160, 971–978. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Response surface methodology (RSM) of chicken skin gelatin based composite films with rice starch and curcumin incorporation. Polym. Test. 2020, 81, 106161. [Google Scholar] [CrossRef]
- Singh, T.P.; Chatli, M.K.; Sahoo, J. Development of chitosan based edible films: Process optimization using response surface methodology. J. Food Sci. Technol. 2014, 52, 2530–2543. [Google Scholar] [CrossRef]
- Thakur, R.; Saberi, B.; Pristijono, P.; Stathopoulos, C.; Golding, J.B.; Scarlett, C.J.; Bowyer, M.; Vuong, Q.V. Use of response surface methodology (RSM) to optimize pea starch–chitosan novel edible film formulation. J. Food Sci. Technol. 2017, 54, 2270–2278. [Google Scholar] [CrossRef]
- ASTM. Annual book of ASTM Standards; D1439-03; American Society for Testing Materials: West Conshohocken, PA, USA, 2005. [Google Scholar]
- ASTM. Standard Test Method for Moisture Absorption Properties and Equilibrium Conditioning of Polymer Matrix Composite Materials; D5229/D5229M-92; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Moisture Content of Paper and Paperboard by Oven Drying (Withdrawn 2010); D644-99; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Water Absorption of Plastics; D570-98; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Cuq, J.L.; Guilber, S. Functional properties of myofibrillar protein based biopackaging as affected by film thickness. J. Food Sci. 1996, 61, 580–584. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Water Vapor Transmission of Materials; E96/E96M-13; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Shiku, Y.; Hamaguchi, P.Y.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of surimi quality on properties of edible films based on Alaska pollack. Food Chem. 2004, 86, 493–499. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Arizmendi-Cotero, D. Optimización de Dos Compuestos Plastificantes (Glicerol y Polietilenglicol) en la Elaboración de Una Película Plástica Comestible Obtenida a Partir del Mucílago de Nopal de la Especie Opuntia Tomentosa Salm-Dyck. Bachelor’s Thesis, Universidad Autónoma del Estado de México, Toluca, Mexico, July 2004. [Google Scholar]
- Herrera-Vázquez, S.E. Efecto de un Recubrimientocomestible a Base de Suero de Leche, Inulina, Grenetina y Glicerol, Sobre las Características Fisicoquímicas, Texturales y Sensoriales de la Fresa (Fragaria vesca L.), Durante su Vida Útil. Master’s Thesis, Universidad Autónoma del Estado de México, Toluca, Mexico, January 2016. [Google Scholar]





| Independent Variables | ||||||
|---|---|---|---|---|---|---|
| Assays | Coded Variables | Uncoded Variables * | ||||
| Gelatin (Factor A) | Whey Protein (Factor B) | Chitosan (Factor C) | Gelatin (g) | Whey Protein (g) | Chitosan (g) | |
| 1 | −1 | 1 | 0 | 3.0 | 7.5 | 1.5 |
| 2 | −1 | 0 | −1 | 3.0 | 5.0 | 0.5 |
| 3 | −1 | 0 | 1 | 3.0 | 5.0 | 2.5 |
| 4 | 1 | 0 | −1 | 6.0 | 5.0 | 0.5 |
| 5 | 1 | 0 | 1 | 6.0 | 5.0 | 2.5 |
| 6 | 0 | −1 | −1 | 4.5 | 2.5 | 0.5 |
| 7 | 0 | −1 | 1 | 4.5 | 2.5 | 2.5 |
| 8 | 1 | 1 | 0 | 6.0 | 7.5 | 1.5 |
| 9 | 0 | 1 | −1 | 4.5 | 7.5 | 0.5 |
| 10 | 0 | 1 | 1 | 4.5 | 7.5 | 2.5 |
| 11 | −1 | −1 | 0 | 3.0 | 2.5 | 1.5 |
| 12 | 1 | −1 | 0 | 6.0 | 2.5 | 1.5 |
| 13 | 0 | 0 | 0 | 3.0 | 5.0 | 1.5 |
| 14 | 0 | 0 | 0 | 3.0 | 5.0 | 1.5 |
| 15 | 0 | 0 | 0 | 3.0 | 5.0 | 1.5 |
| 16 | 0 | 0 | 0 | 3.0 | 5.0 | 1.5 |
| 17 | 0 | 0 | 0 | 3.0 | 5.0 | 1.5 |
| Runs | Responses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V (mPas) | T (mm) | MC (%) | WAC (%) | S (%) | WVP × 10−8 (g/msPa) | TR | L* | a* | b* | ST (N) | YM | |
| 1 | 96.40 | 0.29 | 26.24 | 58.11 | 38.95 | 4.53 | 6.72 | 15.74 | −2.62 | 34.23 | 20.54 | 0.68 |
| (0.40) | (0.02) | (0.93) | (8.98) | (2.36) | (0.01) | (0.03) | (0.06) | (0.05) | (0.36) | (0.38) | (0.01) | |
| 2 | 47.33 | 0.26 | 30.04 | 105.06 | 44.00 | 2.21 | 7.53 | 10.58 | −2.98 | 22.13 | 22.40 | 0.98 |
| (1.22) | (0.01) | (1.76) | (1.08) | (1.85) | (0.00) | (0.04) | (0.18) | (0.02) | (0.62) | (0.30) | (0.09) | |
| 3 | 176.67 | 0.30 | 23.68 | 57.45 | 35.60 | 4.32 | 4.63 | 12.72 | −3.10 | 25.23 | 19.76 | 0.52 |
| (0.23) | (0.01) | (0.17) | (0.34) | (1.13) | (0.01) | (0.09) | (0.09) | (0.05) | (0.41) | (0.28) | (0.05) | |
| 4 | 210.40 | 0.29 | 17.92 | 132.36 | 42.15 | 3.98 | 6.43 | 9.26 | −3.46 | 22.63 | 20.51 | 0.31 |
| (0.85) | (0.00) | (0.26) | (19.02) | (0.53) | (0.01) | (0.01) | (0.08) | (0.02) | (0.29) | (0.17) | (0.02) | |
| 5 | 534.33 | 0.39 | 21.28 | 56.25 | 33.10 | 6.47 | 4.66 | 12.86 | −3.24 | 25.98 | 18.08 | 0.18 |
| (1.30) | (0.01) | (0.74) | (3.79) | (0.83) | (0.02) | (0.05) | (0.50) | (0.07) | (1.18) | (0.14) | (0.00) | |
| 6 | 26.53 | 0.28 | 23.90 | 122.99 | 41.41 | 1.86 | 6.80 | 8.02 | −3.00 | 18.12 | 23.11 | 0.71 |
| (0.15) | (0.01) | (0.40) | (7.34) | (0.87) | (0.00) | (0.03) | (0.11) | (0.04) | (0.55) | (0.49) | (0.01) | |
| 7 | 266.87 | 0.32 | 21.14 | 63.84 | 34.90 | 3.40 | 4.27 | 12.41 | −3.01 | 22.34 | 19.81 | 0.27 |
| (1.60) | (0.00) | (0.67) | (1.47) | (0.37) | (0.01) | (0.03) | (0.21) | (0.12) | (0.54) | (0.29) | (0.01) | |
| 8 | 542.53 | 0.35 | 20.39 | 67.12 | 37.42 | 7.19 | 5.45 | 12.63 | −3.55 | 27.71 | 18.99 | 0.27 |
| (2.50) | (0.01) | (0.86) | (2.73) | (0.32) | (0.02) | (0.03) | (0.12) | (0.01) | (0.36) | (1.12) | (0.02) | |
| 9 | 69.73 | 0.30 | 21.63 | 107.00 | 45.87 | 4.33 | 5.69 | 10.78 | −3.57 | 27.94 | 23.29 | 0.68 |
| (0.23) | (0.01) | (0.15) | (13.98) | (1.59) | (0.00) | (0.00) | (0.18) | (0.01) | (0.34) | (0.65) | (0.03) | |
| 10 | 285.87 | 0.39 | 23.56 | 51.38 | 35.57 | 7.39 | 3.90 | 14.13 | −3.35 | 32.21 | 21.18 | 0.40 |
| (0.23) | (0.01) | (0.28) | (2.28) | (2.67) | (0.01) | (0.02) | (0.21) | (0.06) | (1.53) | (0.19) | (0.02) | |
| 11 | 160.67 | 0.26 | 28.49 | 72.69 | 37.35 | 2.00 | 6.90 | 12.26 | −2.69 | 22.05 | 21.38 | 0.90 |
| (0.83) | (0.00) | (0.41) | (4.74) | (0.43) | (0.01) | (0.01) | (0.47) | (0.06) | (0.86) | (0.38) | (0.01) | |
| 12 | 299.33 | 0.27 | 19.04 | 93.39 | 24.25 | 2.68 | 8.14 | 11.64 | −2.76 | 22.22 | 15.19 | 0.06 |
| (0.61) | (0.01) | (0.23) | (9.93) | (0.53) | (0.01) | (0.05) | (0.56) | (0.07) | (0.40) | (0.70) | (0.01) | |
| 13 | 157.33 | 0.30 | 23.93 | 61.34 | 36.55 | 4.25 | 5.74 | 12.55 | −3.11 | 25.54 | 19.76 | 0.41 |
| (0.61) | (0.01) | (0.89) | (5.24) | (0.94) | (0.01) | (0.02) | (0.16) | (0.04) | (0.10) | (0.46) | (0.03) | |
| 14 | 150.80 | 0.32 | 23.41 | 61.42 | 37.29 | 4.40 | 4.77 | 12.34 | −3.33 | 24.34 | 21.24 | 0.41 |
| (0.40) | (0.00) | (0.20) | (8.12) | (0.71) | (0.01) | (0.03) | (0.40) | (0.04) | (0.85) | (0.27) | (0.02) | |
| 15 | 125.80 | 0.31 | 24.32 | 65.16 | 36.39 | 4.29 | 5.99 | 12.59 | −3.15 | 25.07 | 21.53 | 0.44 |
| (0.72) | (0.00) | (0.88) | (5.30) | (0.84) | (0.01) | (0.06) | (0.27) | (0.08) | (0.67) | (1.15) | (0.01) | |
| 16 | 150.47 | 0.31 | 24.29 | 59.27 | 37.93 | 4.09 | 5.50 | 12.11 | −3.13 | 24.05 | 20.64 | 0.37 |
| (8.73) | (0.00) | (1.25) | (5.86) | (0.70) | (0.01) | (0.01) | (0.14) | (0.02) | (0.05) | (1.35) | (0.08) | |
| 17 | 130.40 | 0.31 | 24.34 | 60.59 | 37.04 | 4.35 | 4.74 | 12.07 | −3.15 | 24.52 | 20.74 | 0.39 |
| (0.40) | (0.02) | (0.59) | (2.29) | (0.46) | (0.01) | (0.41) | (0.26) | (0.07) | (0.40) | (1.00) | (0.06) | |
| Viscosity | Thickness | Moisture Content | Solubility | WAC | WVP (1 × 10−8) | Luminosity | a* Value | b* Value | Transparency | Strength | Young’s Modulus | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Reply (µ) | 142.96 | 0.309 | 24.057 | 37.039 | 61.555 | 4.274 | 12.331 | −3.173 | 24.706 | 5.348 | 0.406 | 41.39 |
| A: Gelatin | 138.192 | 0.024 | −3.727 | −2.372 | 6.976 | 0.907 | −0.615 | −0.203 | −0.636 | −0.139 * | −0.283 | 22.08 |
| AA | 105.853 | −0.016 | 0.079 * | −1.636 | 6.374 | −0.087 * | 0.378 | 0.152 | 0.344 * | 1.052 | 0.026 * | 3.129 |
| B: Whey (g) | 30.142 | 0.024 | −0.091 * | 2.488 | −8.66 | 1.689 | 1.118 | −0.204 | 4.67 | −0.545 | 0.010 * | −5.402 |
| BB | 25.92 | −0.001 | −0.596 | −0.912 | 4.898 | −0.087 * | 0.359 | 0.117 | 1.502 | 0.404 | 0.046 | 0.134 * |
| C: Chitosan (g) | 113.717 | 0.032 | −0.478 | −4.282 | −29.81 | 1.151 | 1.684 | 0.037 | 1.868 | −1.123 | −0.164 | 8.211 |
| CC | −6.630 * | 0.016 | −0.904 | 3.308 | 19.849 | 0.060 * | −1.355 | −0.175 | −1.055 | −0.586 | 0.065 | −3.11 |
| AB | 76.867 | 0.012 | 0.898 | 2.891 | −2.924 * | 0.495 | −0.622 | −0.214 | −1.672 | −0.629 | 0.108 | −11.059 |
| AC | 48.65 | 0.014 | 2.429 | −0.162 * | −7.126 | 0.093 * | 0.366 | 0.085 | 0.064 * | 0.282 * | 0.083 | 0.969 * |
| BC | −6.050 * | 0.011 | 1.174 | −0.949 | 0.883 * | 0.381 | −0.258 | 0.058 * | 0.016 * | 0.184 * | 0.041 | −2.804 |
| Lack of fit (p-value) | 0.37 | 0.545 | 0.163 | 0.002 | 0.048 | 0.748 | 0.129 | 0.892 | 0.061 | 0.999 | 0.624 | 0.573 |
| Pure Error (MS) | 203.3 | 0 | 0.399 | 0.787 | 26.47 | 0.023 | 0.114 | 0.009 | 0.941 | 0.346 | 0.002 | 15.25 |
| R2 | 0.991 | 0.939 | 0.939 | 0.888 | 0.937 | 0.994 | 0.952 | 0.931 | 0.581 | 0.922 | 0.973 | 0.965 |
| CV | 10% | 3% | 3% | 2% | 8% | 4% | 3% | 3% | 4% | 11% | 11% | 9% |
| Responses * | Predicted Value a | Experimental Value (n = 3) b | Absolute Residual Error (%) c |
|---|---|---|---|
| MC (%) | 18.99 | 19.12 ± 0.23 | 0.68 |
| S (%) | 26.81 | 25.25 ± 0.48 | 6.17 |
| TR (%) | 7.86 | 8.21 ± 0.06 | 11.57 |
| YM | 0.079 | 0.065 ± 0.017 | 21.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Vázquez, S.E.; Dublán-García, O.; Arizmendi-Cotero, D.; Gómez-Oliván, L.M.; Islas-Flores, H.; Hernández-Navarro, M.D.; Ramírez-Durán, N. Optimization of the Physical, Optical and Mechanical Properties of Composite Edible Films of Gelatin, Whey Protein and Chitosan. Molecules 2022, 27, 869. https://doi.org/10.3390/molecules27030869
Herrera-Vázquez SE, Dublán-García O, Arizmendi-Cotero D, Gómez-Oliván LM, Islas-Flores H, Hernández-Navarro MD, Ramírez-Durán N. Optimization of the Physical, Optical and Mechanical Properties of Composite Edible Films of Gelatin, Whey Protein and Chitosan. Molecules. 2022; 27(3):869. https://doi.org/10.3390/molecules27030869
Chicago/Turabian StyleHerrera-Vázquez, Selene Elizabeth, Octavio Dublán-García, Daniel Arizmendi-Cotero, Leobardo Manuel Gómez-Oliván, Hariz Islas-Flores, María Dolores Hernández-Navarro, and Ninfa Ramírez-Durán. 2022. "Optimization of the Physical, Optical and Mechanical Properties of Composite Edible Films of Gelatin, Whey Protein and Chitosan" Molecules 27, no. 3: 869. https://doi.org/10.3390/molecules27030869