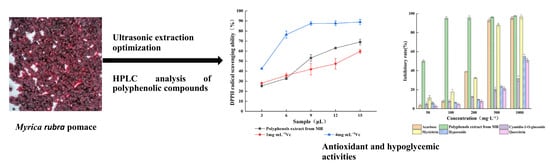
Extraction and Study of Hypoglycemic Constituents from Myrica rubra Pomace
Abstract
:1. Introduction
2. Results
2.1. Single Factor Experimental Results
2.1.1. Effect of Sonication Power on Total Polyphenols Content
2.1.2. Effect of Extraction Time on Total Polyphenols Content
2.1.3. Effect of Solid to Liquid Ratio on Total Polyphenols Content
2.1.4. Effect of Ethanol Concentration on Total Polyphenols Content
2.2. Response Surface Modelling
2.2.1. Analysis of the Response Surface Model
2.2.2. Interactions of Different Experimental Factors on the Response Variables
2.2.3. Validation of Optimal Conditions
2.3. Antioxidant Activity of the Polyphenols Extract from MR Powder
2.4. Liquid Chromatography Analysis
2.5. Analysis of α-Glucosidase Inhibition Experiments
3. Materials and Methods
3.1. Materials
3.2. Chemicals and Solvents
3.3. Extraction Procedure
3.4. Experimental Design and Analytical Methods
3.4.1. Single-Factor Extraction Experiments for Polyphenols MR Powder
3.4.2. Response Surface Experiments for Polyphenols Extraction from MR Powder
3.4.3. Determination of Gallic Acid (GA) Content
3.4.4. Determination of Total Flavonoids
3.4.5. Determination of Total Anthocyanin
3.4.6. Total Phenolic Content Calculation
3.4.7. DPPH Scavenging Capacity
3.4.8. Determination of Hydroxyl Radical Scavenging Ability
3.4.9. Liquid Chromatography Analysis
3.4.10. α-Glucosidase Inhibition Experiment
3.4.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A

References
- Xu, L.N.; Li, Y.; Dai, Y.; Peng, J.Y. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 30, 451–465. [Google Scholar] [CrossRef]
- Wu, Y.L.; Ding, Y.P.; Yoshimasa, T.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.Z.; Zhang, J.Y.; Liu, B.; Yan, T.X.; Xu, F.X.; Xiao, F.; Wu, B.; Bi, K.S.; Jia, Y. Polysaccharide from okra (Abelmoschus esculentus (L.) Moench) improves antioxidant capacity via PI3K/AKT pathways and Nrf2 Translocation in a type 2 diabetes model. Molecules 2019, 24, 1906. [Google Scholar] [CrossRef] [Green Version]
- Mertes, G. Safety and efficacy of acarbose in the treatment of Type 2 diabetes: Data from a 5-year surveillance study. Diabetes Res. Clin. Pract. 2001, 52, 193–204. [Google Scholar] [CrossRef]
- Zhang, M.; Kou, X.J. Analysis of the mechanism of natural products on lowering blood glucose of type 2 diabetes mellitus. Chin. Bull. Life Sci. 2021, 33, 667–676. [Google Scholar]
- van de Laar, F.A.; Lucassen, P.L. α-Glucosidase Inhibitors for Patients with Type 2 Diabetes: Response to Hanefeld et al. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Xie, L.H.; Xie, J.H.; Liu, Y.; Chen, W. Pelargonidin-3-O-rutinoside as a novel α-glucosidase inhibitor for improving postprandial hyperglycemia. Chem. Commun. 2019, 55, 39–42. [Google Scholar] [CrossRef]
- Lee, N.J.; Norris, S.L.; Thakurta, S. Efficacy and harms of the hypoglycemic agent pramlintide in diabetes mellitus. Ann. Fam. Med. 2010, 8, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Sievenpiper, J.L.; Dworatzek, P.D.N. Food and dietary pattern-based recommendations: An emerging approach to clinical practice guidelines for nutrition therapy in diabetes. Can. J. Diabetes 2013, 37, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Arbatskaya, H.Y.; Ignatova, N.G.; Moldovanova, M.V.; Melnikova, E.P.; Kandalina, V.V. The role of dietotherapy in prevention of a diabetic fetopathy at women with gestational diabetes mellitus. J. Obstet. Women’s Dis. 2013, 62, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.N.; Zhu, Y.F.; Ma, H.X.; Wang, X.B.; Zhao, Q.P. The role of traditional Chinese medicine in the treatment of cognitive dysfunction in type 2 diabetes. J. Ethnopharmacol. 2021, 280, 114464. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, S.; Yin, C.P.; Jiang, D.H.; Yan, F.F.; Xu, X. Response surface optimization of aqueous enzymatic oil extraction from bayberry (Myrica rubra) kernels. Food Chem. 2012, 135, 304–308. [Google Scholar] [CrossRef]
- Fang, Z.X.; Bhesh, B. Comparing the efficiency of protein and maltodextrin on spray drying of bayberry juice. Food Res. Int. 2012, 48, 478–483. [Google Scholar] [CrossRef]
- Fang, Z.X.; Zhang, M.; Wang, L.X.; Sun, J.C. Identification of anthocyanin in Bayberry (Myrica rubra Sieb. et Zucc.) by HAPLC-DAD-ESIMS and GC. J. Food Drug Anal. 2006, 14, 8. [Google Scholar] [CrossRef]
- Kim, H.H.; Oh, M.H.; Park, K.J.; Heo, J.H.; Lee, M.W. Anti-inflammatory activity of sulfate-containing phenolic compounds isolated from the leaves of Myrica rubra. Fitoterapia 2014, 92, 188–193. [Google Scholar] [CrossRef]
- Sakurai, N.; Yaguchi, Y.; Inoue, T. Triterpenoids from Myrica rubra. Phytochemistry 1986, 26, 217–219. [Google Scholar] [CrossRef]
- Xia, W.; Gong, E.S.; Lin, Y.Y.; Li, T.; Zheng, B.S.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and antiproliferative activities in Chinese bayberry (Myrica rubra Sieb. et Zucc.) fruits. J. Food Sci. 2021, 86, 4691–4703. [Google Scholar] [CrossRef]
- Lin, K.H.; Lu, C.P.; Chao, J.W.; Yu, Y.P. Antioxidant properties and anti-inflammatory effects of the hydroethanolic extracts of two varieties of bayberry fruit (Myrica rubra Sieb et Zucc.) prepared by stirring and ultrasonic methods. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 634–642. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Li, X.G.; Xiao, Y.B.; Yong, Y.; Zhang, Z.Q.; Li, S.P.; Zhou, T.M.; Yang, D.Q.; Gao, P.C.; Xin, X.L. Hypolipidemic effects of Myrica rubra extracts and main compounds in C57BL/6j mice. Food Funct. 2016, 7, 3505–3515. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wei, C.; Rankin, G.O.; Rojanasakuld, Y.; Ren, N.; Ye, X.Q.; Chen, Y.C. Dietary compound proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves inhibit angiogenesis and regulate cell cycle of cisplatin-resistant ovarian cancer cells via targeting Akt pathway. J. Funct Foods 2018, 40, 573–581. [Google Scholar] [CrossRef]
- Ju, J.; Yao, W.R.; Sun, S.L.; Guo, Y.H.; Cheng, Y.L.; Qian, H.; Xie, Y.F. Assessment of the antibacterial activity and the main bacteriostatic components from bayberry fruit extract. Int. J. Food Prop. 2018, 21, 1043–1051. [Google Scholar] [CrossRef] [Green Version]
- Qua, W.J.; Sehemu, R.M.; Feng, Y.T.; Shi, S.Q.; Wang, J.; Ma, H.L.; Venkitasamy, C. Sonochemical effect of flat sweep frequency and pulsed ultrasound (FSFP) treatment on stability of phenolic acids in a model system. Ultrason. Sonochem. 2017, 39, 707–715. [Google Scholar]
- Sun, Y.J.; Ma, G.P.; Ye, X.Q.; Kakuda, Y.; Meng, R.F. Stability of all-trans-beta-carotene under ultrasound treatment in a model system: Effects of different factors, kinetics and newly formed compounds. Ultrason. Sonochem. 2010, 17, 654–661. [Google Scholar] [CrossRef]
- Wang, P.X.; Cheng, C.X.; Ma, Y.Q.; Jia, M. Degradation behavior of polyphenols in model aqueous extraction system based on mechanical and sonochemical effects induced by ultrasound. Sep. Purif. Technol. 2020, 247, 116967. [Google Scholar] [CrossRef]
- Brglez, M.E.; Knez, H.M.; Škerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, H.L.; Xu, Z.B.; Zheng, B.D.; Lin, Y.X.; Gan, C.J.; Lo, Y.M. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus). Carbohyd. Polym. 2012, 88, 522–529. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Zhu, Y.P.; Jiao, C.Y.; Shi, M.Y.; Wei, L.P.; Zhou, Y.; Jin, Q.; Cai, Y.P. Extraction and analysis of gigantol from Dendrobium officinale with response surface methodology. Molecules 2018, 23, 818. [Google Scholar] [CrossRef] [Green Version]
- Schmid, R. Recent advances in the description of the structure of water, the hydrophobic effect, and the like-dissolves-like rule. Mon. für Chem. 2001, 132, 1295–1326. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Hayat, K.; Hussain, S.; Abbas, S.; Farooq, U.; Ding, B.M.; Xia, S.Q.; Jia, C.S.; Zhang, X.M.; Xia, W.S. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep. Purif. Technol. 2009, 70, 63–70. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, E.; Yi, C.; Zhao, M.M.; Jiang, Y.M. Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. Innov. Food Sci Emerg. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Morales, P.; Ferreira, I.C.F.R. Floral parts of Gomphrena globosa L. as a novel alternative source of betacyanins: Optimization of the extraction using response surface methodology. Food Chem. 2017, 229, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senejoux, F.; Demougeot, C.; Kerram, P.; Aisa, H.A.; Berthelot, A.; Bévalot, F.; Girard-Thernier, C. Bioassay-guided isolation of vasorelaxant compounds from Ziziphora clinopodioides Lam. (Lamiaceae). Fitoterapia 2012, 83, 377–382. [Google Scholar] [CrossRef]
- Hossain, M.A.; Rahman, S.M.M. Rahman Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple. Food Res. Int. 2011, 44, 672–676. [Google Scholar] [CrossRef]
- Wang, W.D.; Xu, S.Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Zheng, L.Q.; Ding, Z.S.; Zhang, M.; Sun, J.C. Microencapsulation of bayberry polyphenols by ethyl cellulose: Preparation and characterization. J. Food Eng. 2011, 104, 89–95. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.Y.; He, Z.Q.; Chen, Y.J.; Li, Z.Y.; Meng, T.M.; Li, Y.F.; Cao, Y. In vitro and in vivo antioxidant activity of eucalyptus leaf polyphenols extract and its effect on chicken meat quality and cecum microbiota. Food Res. Int. 2020, 136, 109302. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G.X. Antioxidant and α-glucosidase inhibitory activity of colored grains in China. J. Agric. Food. Chem. 2010, 58, 770–774. [Google Scholar] [CrossRef]









| Number | Ethanol Concentration (%) | Time (min) | Solid-to-Liquid Ratio | Total Polyphenols Content (mg·g−1) |
|---|---|---|---|---|
| 1 | 20 (−1) | 70 (1) | 1:20 (−1) | 14.0994 |
| 2 | 40 (0) | 16.36 (−1.682) | 1:30 (0) | 19.5022 |
| 3 | 40 (0) | 50 (0) | 1:30 (0) | 23.9611 |
| 4 | 40 (0) | 50 (0) | 1:30 (0) | 23.0864 |
| 5 | 73.64 (1.682) | 50 (0) | 1:30 (0) | 20.1641 |
| 6 | 40 (0) | 50 (0) | 1:30 (0) | 21.224 |
| 7 | 6.36 (−1.682) | 50 (0) | 1:30 (0) | 14.7639 |
| 8 | 20 (−1) | 30 (−10) | 1:20 (−1) | 13.8511 |
| 9 | 60 (1) | 70 (1) | 1:40 (1) | 22.1833 |
| 10 | 40 (0) | 50 (0) | 1:30 (0) | 23.3016 |
| 11 | 60 (1) | 70 (1) | 1:20 (−1) | 19.0647 |
| 12 | 60 (1) | 30 (−1) | 1:40 (1) | 21.0816 |
| 13 | 40 (0) | 50 (0) | 1: 46.82 (1.682) | 20.6164 |
| 14 | 40 (0) | 50 (0) | 1: 13.18 (−1.682) | 15.8053 |
| 15 | 20 (−1) | 30 (−1) | 1:40 (1) | 14.795 |
| 16 | 20 (−1) | 70 (1) | 1:40 (10 | 12.971 |
| 17 | 40 (0) | 50 (0) | 1:30 (0) | 21.5682 |
| 18 | 60 (1) | 30 (−1) | 1:20 (−1) | 18.1948 |
| 19 | 40 (0) | 50 (0) | 1:30 (0) | 22.8778 |
| 20 | 40 (0) | 83.64 (1.682) | 1:30 (0) | 22.6394 |
| Source | Quadratic Sum | Degrees of Freedom | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| df | Prob > F | |||||
| Model | 223.38 | 9 | 24.82 | 9.31 | 0.0009 | ** |
| A-Ethanol concentration | 84.1 | 1 | 84.1 | 31.55 | 0.0002 | ** |
| B-Time | 2.36 | 1 | 2.36 | 0.88 | 0.3693 | |
| C-Solid-to-liquid ratio | 14.17 | 1 | 14.17 | 5.32 | 0.0438 | * |
| AB | 1.57 | 1 | 1.57 | 0.59 | 0.4601 | |
| AC | 4.79 | 1 | 4.79 | 1.8 | 0.2098 | |
| BC | 0.42 | 1 | 0.42 | 0.16 | 0.6986 | |
| A2 | 69.14 | 1 | 69.14 | 25.94 | 0.0005 | ** |
| B2 | 12.07 | 1 | 12.07 | 4.53 | 0.0592 | |
| C2 | 53.47 | 1 | 53.47 | 20.06 | 0.0012 | ** |
| Residual error | 26.66 | 10 | 2.67 | |||
| Lack of fit | 21.07 | 5 | 4.21 | 3.77 | 0.0858 | N |
| Pure error | 5.59 | 5 | 1.12 | |||
| Total value | 250.03 | 19 |
| Factors | Code | Level | ||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | ||
| Ethanol concentration (%) | A | 6.36 | 20 | 40 | 60 | 73.64 |
| Time (min) | B | 16.36 | 30 | 50 | 70 | 83.64 |
| Solid-to-liquid ratio | C | 1:13.18 | 1:20 | 1:30 | 1:40 | 1:46.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, G.; Cai, C.; Xiang, Y.; Fang, X.; Yang, H. Extraction and Study of Hypoglycemic Constituents from Myrica rubra Pomace. Molecules 2022, 27, 846. https://doi.org/10.3390/molecules27030846
Chang G, Cai C, Xiang Y, Fang X, Yang H. Extraction and Study of Hypoglycemic Constituents from Myrica rubra Pomace. Molecules. 2022; 27(3):846. https://doi.org/10.3390/molecules27030846
Chicago/Turabian StyleChang, Guoli, Chenggang Cai, Yannan Xiang, Xiangjun Fang, and Hailong Yang. 2022. "Extraction and Study of Hypoglycemic Constituents from Myrica rubra Pomace" Molecules 27, no. 3: 846. https://doi.org/10.3390/molecules27030846