Autoxidation Products of the Methanolic Extract of the Leaves of Combretum micranthum Exert Antiviral Activity against Tomato Brown Rugose Fruit Virus (ToBRFV)
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis of the Autoxidized Methanolic Extract of the Leaves of C. micranthum
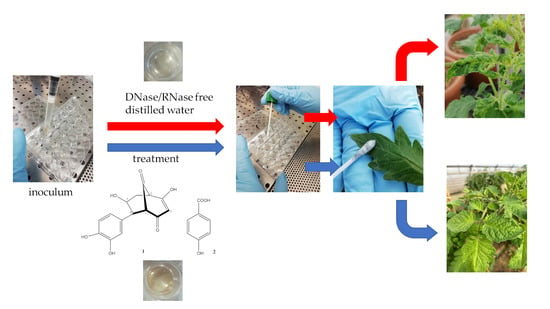
2.2. Antiviral Activity Assay and Evaluation of Final Viability of the Virus
2.3. Homology Modelling and Validation
2.4. Molecular Docking Studies
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. General Experimental Procedures
4.3. Plant Material
4.4. Preparation of Alkaline Autoxidized Methanolic Extract and Purification
4.5. Preparation of Catechinic Acid and of Its Alkaline Autoxidation Product
4.6. Antiviral Activity Assay
4.7. Plant Inoculation
4.8. RT-PCR for the Detection of the Virus
4.9. Molecular Modeling Studies
4.9.1. Homology Modelling and Protein Preparation
4.9.2. Ligands Preparation
4.9.3. Docking Studies
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pennazio, S.; Roggero, P.; Conti, M. Yield losses in virus-infected crops. Arch. Phytopathol. Plant Prot. 1996, 30, 283–296. [Google Scholar] [CrossRef]
- Rubio, L.; Galipienso, L.; Ferriol, I. Detection of plant viruses and disease management: Relevance of genetic diversity and evolution. Front. Plant Sci. 2020, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, P.A.; Baleev, D.N.; Ivanova, M.I.; Sokolova, L.M.; Karakozova, M.V. Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Naturae 2020, 12, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.C. Global plant virus disease pandemics and epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef]
- Chauhan, P.; Singla, K.; Rajbhar, M.; Singh, A.; Das, N.; Kumar, K. A systematic review of conventional and advanced approaches for the control of plant viruses. J. Appl. Biol. Biotechnol. 2019, 7, 89–98. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2019. Moving forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- Yan, Z.-Y.; Zhao, M.-S.; Ma, H.-Y.; Liu, L.-Z.; Yang, G.-L.; Geng, C.; Tian, Y.; Li, X.-D. Biological and molecular characterization of tomato brown rugose fruit virus and development of quadruplex RT-PCR detection. J. Integr. Agric. 2021, 20, 1871–1879. [Google Scholar] [CrossRef]
- Chanda, B.; Shamimuzzaman, M.; Gilliard, A.; Ling, K.-S. Effectiveness of disinfectants against the spread of tobamoviruses: Tomato brown rugose fruit virus and Cucumber green mottle mosaic virus. Virol. J. 2021, 18, 7. [Google Scholar] [CrossRef]
- Scholthof, K.B. Tobacco mosaic virus: A model system for plant biology. Annu. Rev. Phytopathol. 2004, 42, 13–34. [Google Scholar] [CrossRef]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.W.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef]
- Menzel, W.; Knierim, D.; Winter, S.; Hamacher, J.; Heupel, M. First report of Tomato brown rugose fruit virus infecting tomato in Germany. New Dis. Rep. 2019, 39, 2044-0588. [Google Scholar] [CrossRef]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A new Israeli tobamovirus isolate infects tomato plants harboring Tm-22 resistance genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef]
- Panno, S.; Ruiz-Ruiz, S.; Caruso, A.G.; Alfaro-Fernandez, A.; Font San Ambrosio, M.I.; Davino, S. Real-time reverse transcription polymerase chain reaction development for rapid detection of Tomato brown rugose fruit virus and comparison with other techniques. PeerJ 2019, 7, e7928. [Google Scholar] [CrossRef]
- Camacho-Beltrán, E.; Pérez-Villarreal, A.; Leyva-López, N.E.; Rodríguez-Negrete, E.A.; Ceniceros-Ojeda, E.A.; Méndez-Lozano, J. Occurrence of Tomato brown rugose fruit virus infecting tomato crops in Mexico. Plant Dis. 2019, 103, 1440. [Google Scholar] [CrossRef]
- Alkowni, R.; Alabdallah, O.; Fadda, Z. Molecular identification of Tomato brown rugose fruit virus in tomato in Palestine. J. Plant Pathol. 2019, 101, 719–723. [Google Scholar] [CrossRef]
- Fidan, H.; Sarıkaya, P.; Calis, O. First report of Tomato brown rugose fruit virus on tomato in Turkey. New Dis. Rep. 2019, 39, 18. [Google Scholar] [CrossRef]
- Skelton, A.; Buxton-Kirk, A.; Ward, R.; Harju, V.; Frew, L.; Fowkes, A.; Long, M.; Negus, A.; Forde, S.; Adams, I.P.; et al. First report of Tomato brown rugose fruit virus in tomato in the United Kingdom. New Dis. Rep. 2019, 40, 12. [Google Scholar] [CrossRef]
- Ling, K.-S.; Tian, T.; Gurung, S.; Salati, R.; Gilliard, A. First report of Tomato brown rugose fruit virus infecting greenhouse tomato in the United States. Plant Dis. 2019, 103, 1439. [Google Scholar] [CrossRef]
- Broadbent, L.; Fletcher, J.T. The epidemiology of tomato mosaic. IV. Persistence of virus on clothing and glasshouse structures. Ann. Appl. Biol. 1963, 52, 233–241. [Google Scholar] [CrossRef]
- Gülser, C.; Yilmaz, N.D.K.; Candemir, F. Accumulation of Tobacco mosaic virus (TMV) at different depths clay and loamy sand textural soils due to tobacco waste application. Environ. Monit. Assess. 2008, 146, 235–242. [Google Scholar] [CrossRef]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef]
- Jones, R.A.C. Control of plant virus diseases. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2006; Volume 67, pp. 205–244. [Google Scholar]
- Brühl, C.A.; Zaller, J.G. Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Front. Environ. Sci. 2019, 7, 177. [Google Scholar] [CrossRef]
- Assey, G.E.; Mgohamwende, R.; Malasi, W.S. A review of the impact of pesticides pollution on environment including effects, benefits and control. J. Pollut. Eff. Cont. 2021, 9, 282. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Fraternale, D.; Giacomini, M.; Giacomelli, E.; Pivetti, S.; Russo, E.; Caviglioli, G.; Romussi, G.; Ricci, D.; De Tommasi, N. Phytotoxicity of Salvia spp. exudates. Crop. Prot. 2010, 29, 1434–1446. [Google Scholar] [CrossRef]
- Bisio, A.; Damonte, G.; Fraternale, D.; Giacomelli, E.; Salis, A.; Romussi, G.; Cafaggi, S.; Ricci, D.; De Tommasi, N. Phytotoxic clerodane diterpenes from Salvia miniata Fernald (Lamiaceae). Phytochemistry 2011, 72, 265–275. [Google Scholar] [CrossRef]
- Bisio, A.; Fraternale, D.; Damonte, G.; Millo, E.; Lanteri, A.P.; Russo, E.; Romussi, G.; Parodi, B.; Ricci, D.; De Tommasi, N. Phytotoxic activity of Salvia x jamensis. Nat. Prod. Commun. 2009, 4, 1621–1630. [Google Scholar] [CrossRef]
- Parizipour, M.H.G.; Shahriari, A.G. Investigation of antiviral potential of licorice (Glycyrrhiza glabra L.) crude extract against Tobacco mosaic virus. J. Anim. Plant Sci. 2020, 30, 107–114. [Google Scholar] [CrossRef]
- Bezj, N.; Dunkj, V.; Vuko, E. Antiphytoviral activity of essential oils of some Lamiaceae species and there most important compounds on CMV and TMV. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Microbiology Book Series; Formatex Research Center: Badajoz, Spain, 2013; pp. 982–988. [Google Scholar]
- Emamzadeh Yazdi, S.; Mulabisana, J.; Prinsloo, G.; Cloete, M.; Kritzinger, Q. Plants containing cardiac glycosides showing antiphytoviral activity against Potato virus Y (PVYNTN) on tobacco plants. J. Plant. Prot. Res. 2018, 58, 397–403. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef]
- Dadáková, K.; Heinrichová, T.; Lochman, J.; Kašparovský, T. Production of defense phenolics in tomato leaves of different age. Molecules 2020, 25, 4952. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, T. Antimicrobial activities of tea polyphenol on phytopathogens: A review. Molecules 2019, 24, 816. [Google Scholar] [CrossRef] [PubMed]
- Gillmeister, M.; Ballert, S.; Raschke, A.; Geistlinger, J.; Kabrodt, K.; Baltruschat, H.; Deising, H.B.; Schellenberg, I. Polyphenols from Rheum roots inhibit growth of fungal and Oomycete phytopathogens and induce plant disease resistance. Plant Dis. 2019, 103, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-F.; Chik, I.W.; Wang, D.-Y.; Pan, L.-T. Plant phenolic compounds as potential lead compounds in functional foods for antiviral drug discovery. Curr. Org. Chem. 2017, 21, 1847–1860. [Google Scholar] [CrossRef]
- Song, P.; Yu, X.; Yang, W.; Wang, Q. Natural phytoalexin stilbene compound resveratrol and its derivatives as anti-Tobacco mosaic virus and anti-phytopathogenic fungus agents. Sci. Rep. 2021, 11, 16509. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Li, X.; Yin, L.; Jiang, D.; Hu, D. Design, synthesis, antiviral bioactivity, and mechanism of the ferulic acid ester-containing sulfonamide moiety. ACS Omega 2020, 5, 19721–19726. [Google Scholar] [CrossRef]
- Hu, Z.-X.; Zou, J.-B.; An, Q.; Yi, P.; Yuan, C.-M.; Gu, W.; Huang, L.-J.; Lou, H.-Y.; Zhao, L.-H.; Hao, X.-J. Anti-Tobacco mosaic virus (TMV) activity of chemical constituents from the seeds of Sophora tonkinensis. J. Asian Nat. Prod. Res. 2021, 23, 644–651. [Google Scholar] [CrossRef]
- Welch, C.; Zhen, J.; Bassène, E.; Raskin, I.; Simon, J.E.; Wu, Q. Bioactive polyphenols in kinkéliba tea (Combretum micranthum) and their glucose-lowering activities. J. Food Drug Anal. 2018, 26, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Welch, C.; Guo, Y.; Bassène, E.; Raskin, I.; Simon, J.E.; Wu, Q. Novel skeleton flavan-alkaloids from African herb tea kinkéliba: Isolation, characterization, semisynthesis, and bioactivities. In African Natural Plant Products, Volume III: Discoveries and Innovations in Chemistry, Bioactivity, and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1361, pp. 297–312. [Google Scholar]
- D’Agostino, M.; Biagi, C.; De Feo, V.; Zollo, F.; Pizza, C. Flavonoids of Combretum micranthum. Fitoterapia 1990, 61, 477. [Google Scholar]
- Tine, D.; Fall, A.D.; Mbacké Dieng, S.I.; Sarr, A.; Bassene, E. Total polyphenol, tannin and flavonoid contents of Combretum micranthum leaves harvested in three regions of Senegal: Diass, Sandiara and Essyl. Int. J. Biol. Chem. Sci. 2019, 13, 1817–1820. [Google Scholar] [CrossRef]
- Bassène, E.O.; Olschwang, D.; Pousset, J.L. Plantes medicinales Africaines. Les alcaloides du Combretum micranthum G. Don (Kinkeliba). Ann. Pharm. Fr. 1986, 44, 191–196. [Google Scholar] [PubMed]
- Bassene, E.; Olschwang, D.; Pousset, J. Plantes medicinales africaines I: Caracterisation de l’inositol et du sorbitol principes actifs probables du Kinkeliba (Combretum micranthum G.Don.). Dakar Med. 1981, 26, 219–225. [Google Scholar] [PubMed]
- Ferrea, G.; Canessa, A.; Sampietro, F.; Cruciani, M.; Romussi, G.; Bassetti, D. In vitro activity of a Combretum micranthum extract against Herpes simplex virus types 1 and 2. Antivir. Res. 1993, 21, 317–325. [Google Scholar] [CrossRef]
- El Sayed, K.A. Natural products as antiviral agents. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 24, pp. 473–572. [Google Scholar]
- Sears, K.D.; Casebier, R.L.; Hergert, H.L.; Stout, G.H.; McCandlish, L.E. Structure of catechinic acid. Base rearrangement product of catechin. J. Org. Chem. 1974, 39, 3244–3247. [Google Scholar] [CrossRef]
- Okada, F.; Takeo, T.; Okada, S.; Tamemasa, O. Antiviral effect of theaflavins on Tobacco mosaic virus. Agric. Biol. Chem. 1977, 41, 791–794. [Google Scholar]
- Okada, F. Antiviral effects of tea catechins and black tea theaflavins on plant viruses. JARQ 1978, 12, 27–32. [Google Scholar]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First report of protective activity of Paronychia argentea extract against Tobacco mosaic virusi infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef]
- Zhang, X.N.; Liao, Y.W.; Wang, X.R.; Zhang, L.; Ahammed, G.J.; Li, Q.Y.; Li, X. Epigallocatechin-3-gallate enhances tomato resistance to Tobacco mosaic virus by modulating RBOH1-dependent H2O2 signaling. Plant Physiol. Biochem. 2020, 150, 263–269. [Google Scholar] [CrossRef]
- Reybrouck, G. The testing of disinfectants. Int. Biodeterior. Biodegrad. 1998, 41, 269–272. [Google Scholar] [CrossRef]
- Hull, R. Mechanical inoculation of plant viruses. Curr. Protoc. Microbiol. 2009, 13, 16B.16.11–16B.16.14. [Google Scholar] [CrossRef]
- Klug, A. The Tobacco mosaic virus particle: Structure and assembly. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999, 354, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, G.; Wang, Q.; Chen, Z.; Ding, Y.; Yu, L.; Hu, D.; Song, B. Ningnanmycin inhibits Tobacco mosaic virus virulence by binding directly to its coat protein discs. Oncotarget 2017, 8, 82446–82458. [Google Scholar] [CrossRef] [PubMed]
- Callaway, A.; Giesman-Cookmeyer, D.; Gillock, E.T.; Sit, T.L.; Lommel, S.A. The multifunctional capsid proteins of plant RNA viruses. Annu. Rev. Phytopathol. 2001, 39, 419–460. [Google Scholar] [CrossRef] [PubMed]
- Bhyravbhatla, B.; Watowich, S.J.; Caspar, D.L. Refined atomic model of the four-layer aggregate of the Tobacco mosaic virus coat protein at 2.4-A resolution. Biophys. J. 1998, 74, 604–615. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Ehrenfeld, N.; Gonzalez, A.; Cañón, P.; Medina, C.; Perez-Acle, T.; Arce-Johnson, P. Structure-function relationship between the tobamovirus TMV-Cg coat protein and the HR-like response. J. Gen. Virol. 2008, 89, 809–817. [Google Scholar] [CrossRef]
- Guo, X.; Li, W.-X.; Lu, R. Silencing of host genes directed by virus-derived short interfering RNAs in Caenorhabditis elegans. J. Virol. 2012, 86, 11645–11653. [Google Scholar] [CrossRef]
- Dawson, W.O. Tobamovirus-plant interactions. Virology 1992, 186, 359–367. [Google Scholar] [CrossRef]
- Dawson, W.O.; Bubrick, P.; Grantham, G.L. Modifications of the Tobacco mosaic virus coat protein gene affecting replication, movement, and symptomatology. Phytopathology 1988, 78, 783–789. [Google Scholar] [CrossRef]
- Novikov, V.K.; Belenovich, E.V.; Dobrov, E.N.; Zavriev, S.K. Kazakh strains of Tobacco mosaic virus: Two strains with potentially destabilizing amino acid substitutions in the coat protein. Physiol. Mol. Plant Pathol. 2000, 56, 71–77. [Google Scholar] [CrossRef]
- Lu, B.; Taraporewala, F.; Stubbs, G.; Culver, J.N. Intersubunit interactions allowing a carboxylate mutant coat protein to inhibit tobamovirus disassembly. Virology 1998, 244, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Asurmendi, S.; Berg, R.H.; Smith, T.J.; Bendahmane, M.; Beachy, R.N. Aggregation of TMV CP plays a role in CP functions and in coat-protein-mediated resistance. Virology 2007, 366, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Culver, J.N. Tobacco mosaic virus assembly and disassembly: Determinants in pathogenicity and resistance. Annu. Rev. Phytopathol. 2002, 40, 287–308. [Google Scholar] [CrossRef]
- Bloomer, A.C.; Champness, J.N.; Bricogne, G.; Staden, R.; Klug, A. Protein disk of Tobacco mosaic virus at 2.8 Å resolution showing the interactions within and between subunits. Nature 1978, 276, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Namba, K.; Pattanayek, R.; Stubbs, G. Visualization of protein-nucleic acid interactions in a virus. Refined structure of intact Tobacco mosaic virus at 2.9 A resolution by X-ray fiber diffraction. J. Mol. Biol. 1989, 208, 307–325. [Google Scholar] [CrossRef]
- Stubbs, G.; Warren, S.; Holmes, K. Structure of RNA and RNA binding site in Tobacco mosaic virus from 4-Å map calculated from X-ray fibre diagrams. Nature 1977, 267, 216–221. [Google Scholar] [CrossRef]
- Sharma, J.; Purohit, R.; Hallan, V. Conformational behavior of coat protein in plants and association with coat protein-mediated resistance against TMV. Braz. J. Microbiol. 2020, 51, 893–908. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Liu, T.; Mou, H.; Wei, C.; Hu, D.; Song, B. Design, synthesis, and antiviral activities of coumarin derivatives containing dithioacetal structures. J. Agric. Food Chem. 2020, 68, 975–981. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Jabłońska, J.; Pravda, L.; Vařeková, R.S.; Thornton, J.M. PDBsum: Structural summaries of PDB entries. Protein Sci. 2018, 27, 129–134. [Google Scholar] [CrossRef]
- Oladokun, J.O.; Halabi, M.H.; Barua, P.; Nath, P.D. Tomato brown rugose fruit disease: Current distribution, knowledge and future prospects. Plant Pathol. 2019, 68, 1579–1586. [Google Scholar] [CrossRef]
- Maayan, Y.; Pandaranayaka, E.P.J.; Srivastava, D.A.; Lapidot, M.; Levin, I.; Dombrovsky, A.; Harel, A. Using genomic analysis to identify tomato Tm-2 resistance-breaking mutations and their underlying evolutionary path in a new and emerging tobamovirus. Arch. Virol. 2018, 163, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Georgousaki, K.; Tsafantakis, N.; Gumeni, S.; Lambrinidis, G.; González-Menéndez, V.; Tormo, J.R.; Genilloud, O.; Trougakos, I.P.; Fokialakis, N. Biological evaluation and in silico study of benzoic acid derivatives from Bjerkandera adusta targeting proteostasis network modules. Molecules 2020, 25, 666. [Google Scholar] [CrossRef] [PubMed]
- Honma, K.; Kuroiwa, Y.; Hamada, A. Aliphatic dehydroxylation of octopamine in the rat and rabbit. Chem. Pharm. Bull. (Tokyo) 1975, 23, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Kiatgrajai, P.; Wellons, J.D.; Gollob, L.; White, J.D. Kinetics of epimerization of (+)-catechin and its rearrangement to catechinic acid. J. Org. Chem. 1982, 47, 2910–2912. [Google Scholar] [CrossRef]
- Hashida, K.; Ohara, S. Formation of a novel catechinic acid stereoisomer from base-catalyzed reactions of (+)-catechin. J. Wood Chem. Technol. 2002, 22, 11–23. [Google Scholar] [CrossRef]
- Rodríguez-Mendoza, J.; García-Ávila, C.D.J.; López-Buenfil, J.A.; Araujo-Ruiz, K.; Quezada-Salinas, A.; Cambrón-Crisantos, J.M.; Ochoa-Martínez, D.L. Identificación de Tomato brown rugose fruit virus por RT-PCR de una región codificante de la replicasa (RdRP). Rev. Mex. Fitopatol. 2019, 37, 345–356. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2020, 49, D480–D489. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- RCSB PDB. RCSB protein data bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef]
- Schrödinger. Schrödinger Release 2020: Maestro; Schrödinger, LLC.: New York, NY, USA, 2020. [Google Scholar]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Schrödinger. Schrödinger Release 2021-4: Prime; Schrödinger, LLC.: New York, NY, USA, 2021. [Google Scholar]
- Sharma, J.; Bhardwaj, V.K.; Das, P.; Purohit, R. Plant-based analogues identified as potential inhibitor against Tobacco mosaic virus: A biosimulation approach. Pestic. Biochem. Physiol. 2021, 175, 104858. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, D.; Gan, X.; Zeng, S.; Zhang, A.; Yin, L.; Song, B.; Jin, L.; Hu, D. Synthesis, antiviral activity, and molecular docking study of trans-ferulic acid derivatives containing acylhydrazone moiety. Bioorg. Med. Chem. Lett. 2017, 27, 4096–4100. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zan, N.; Huang, M.; Jiang, D.; Hu, D.; Song, B. Design, synthesis and anti-TMV activities of novel chromone derivatives containing dithioacetal moiety. Bioorg. Med. Chem. Lett. 2020, 30, 126945. [Google Scholar] [CrossRef]
- Cassells, A.C. Chemical control of virus diseases of plants. Prog. Med. Chem. 1983, 20, 119–155. [Google Scholar] [CrossRef]
- Lozoya-Saldana, H.; Dawson, W.O.; Murashige, T. Effects of ribavirin and adenine arabinoside on Tobacco mosaic virus in Nicotiana tabacum L. var Xanthi tissue cultures. Plant Cell Tiss. Org. Cult. 1984, 3, 41–48. [Google Scholar] [CrossRef]
- Wang, Y.; He, F.; Wu, S.; Luo, Y.; Wu, R.; Hu, D.; Song, B. Design, synthesis, anti-TMV activity, and preliminary mechanism of cinnamic acid derivatives containing dithioacetal moiety. Pestic. Biochem. Physiol. 2020, 164, 115–121. [Google Scholar] [CrossRef]
- Wei, C.; Zhao, L.; Sun, Z.; Hu, D.; Song, B. Discovery of novel indole derivatives containing dithioacetal as potential antiviral agents for plants. Pestic. Biochem. Physiol. 2020, 166, 104568. [Google Scholar] [CrossRef]
- Yang, S.; Wang, T.; Zhou, Y.; Shi, L.; Lu, A.; Wang, Z. Discovery of cysteine and its derivatives as novel antiviral and antifungal agents. Molecules 2021, 26, 383. [Google Scholar] [CrossRef]
- Zhou, D.; Xie, D.; He, F.; Song, B.; Hu, D. Antiviral properties and interaction of novel chalcone derivatives containing a purine and benzenesulfonamide moiety. Biorg. Med. Chem. Lett. 2018, 28, 2091–2097. [Google Scholar] [CrossRef]
- Germann, D.; Stark, T.D.; Hofmann, T. Formation and characterization of polyphenol-derived red chromophores. Enhancing the color of processed cocoa powders: Part 1. J. Agric. Food Chem. 2019, 67, 4632–4642. [Google Scholar] [CrossRef]
- Laks, P.E.; Hemingway, R.W.; Conner, A.H. Condensed tannins. Base-catalysed reactions of polymeric procyanidins with phloroglucinol: Intramolecular rearrangements. J. Chem. Soc. Perkin Trans. 1 1987, 1875–1881. [Google Scholar] [CrossRef]
- Ohara, S.; Hemingway, R.W. Condensed tannins: The formation of a diarylpropanol-catechinic acid dimer from base-catalyzed reactions of (+)-catechin. J. Wood Chem. Technol. 1991, 11, 195–208. [Google Scholar] [CrossRef]
- Newhall, W.F.; Ting, S.V. Degradation of hesperetin and naringenin to phloroglucinol. J. Agric. Food Chem. 1967, 15, 776–777. [Google Scholar] [CrossRef]
- Niklas, K.J.; Giannasi, D.E. Geochemistry and thermolysis of flavonoids. Science 1977, 197, 767–769. [Google Scholar] [CrossRef]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Yew, P.Y.M.; Owh, C.; Chee, P.L.; Loh, X.J. Sanitizing agents for virus inactivation and disinfection. View 2020, 1, e16. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhou, K.; Chen, G.; Wang, Q. Self-assembly of protein crystals with different crystal structures using Tobacco mosaic virus coat protein as a building block. ACS Nano 2018, 12, 1673–1679. [Google Scholar] [CrossRef]
- Xia, R.; Guo, T.; Chen, M.; Su, S.; He, J.; Tang, X.; Jiang, S.; Xue, W. Synthesis, antiviral and antibacterial activities and action mechanism of penta-1,4-dien-3-one oxime ether derivatives containing a quinoxaline moiety. New J. Chem. 2019, 43, 16461–16467. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, C.; Chen, M.; Xue, Y.; Liu, T.; Xue, W. Synthesis and antiviral activity of novel myricetin derivatives containing ferulic acid amide scaffolds. New J. Chem. 2020, 44, 2374–2379. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Su, S.; Chen, M.; He, J.; Liu, L.; He, M.; Wang, H.; Xue, W. Synthesis and antibacterial and antiviral activities of myricetin derivatives containing a 1,2,4-triazole Schiff base. RSC Adv. 2019, 9, 23045–23052. [Google Scholar] [CrossRef]
- Alon, D.M.; Hak, H.; Bornstein, M.; Pines, G.; Spiegelman, Z. Differential detection of the tobamoviruses Tomato mosaic virus (ToMV) and Tomato brown rugose fruit virus (ToBRFV) using CRISPR-Cas12a. Plants 2021, 10, 1256. [Google Scholar] [CrossRef]
- Butler, P.J.; Klug, A. Assembly of Tobacco mosaic virus in vitro: Effect of state of polymerization of the protein component. Proc. Natl. Acad. Sci. USA 1972, 69, 2950–2953. [Google Scholar] [CrossRef]
- Li, X.-Y.; Song, B.-A. Progress in the development and application of plant-based antiviral agents. J. Integr. Agric. 2017, 16, 2772–2783. [Google Scholar] [CrossRef]
- Purohit, R.; Kumar, S.; Hallan, V. Screening of potential inhibitor against coat protein of Apple chlorotic leaf spot virus. Cell Biochem. Biophys. 2018, 76, 273–278. [Google Scholar] [CrossRef]
- Guo, W.; Lu, X.; Liu, B.; Yan, H.; Feng, J. Anti-TMV activity and mode of action of three alkaloids isolated from Chelidonium majus. Pest Manage. Sci. 2021, 77, 510–517. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, J.; Hu, Z.; Li, S.; Su, X.; Zhang, J.; Yin, Y.; Xu, T.; Zhang, Z.; Chen, H. Anti-TMV activity and functional mechanisms of two sesquiterpenoids isolated from Tithonia diversifolia. Pestic. Biochem. Physiol. 2017, 140, 24–29. [Google Scholar] [CrossRef]
- Chen, J.; Yan, X.-H.; Dong, J.-H.; Sang, P.; Fang, X.; Di, Y.-T.; Zhang, Z.-K.; Hao, X.-J. Tobacco mosaic virus (TMV) inhibitors from Picrasma quassioides Benn. J. Agric. Food Chem. 2009, 57, 6590–6595. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Guo, D.-S.; Lu, M.-H.; Yue, J.-Y.; Liu, Y.; Shang, C.-M.; An, D.-R.; Zhao, M.-M. Inhibitory effect of osthole from Cnidium monnieri on Tobacco mosaic virus (TMV) infection in Nicotiana glutinosa. Molecules 2020, 25, 65. [Google Scholar] [CrossRef]
- Min, L.; Zhiqiang, H.; Yun, X. In vitro and in vivo anti-Tobacco mosaic virus activities of essential oils and individual compounds. J. Microbiol. Biotechnol. 2013, 23, 771–778. [Google Scholar] [CrossRef]
- Islam, W.; Qasim, M.; Noman, A.; Tayyab, M.; Chen, S.; Wang, L. Management of Tobacco mosaic virus through natural metabolites. Rec. Nat. Prod. 2018, 12, 403–415. [Google Scholar] [CrossRef]
- Li, J.-X.; Liu, S.-S.; Gu, Q. Transmission efficiency of Cucumber green mottle mosaic virus via seeds, soil, pruning and irrigation water. J. Phytopathol. 2016, 164, 300–309. [Google Scholar] [CrossRef]
- Smith, E.; Luria, N.; Reingold, V.; Frenkel, O.; Koren, A.; Klein, E.; Bekelman, H.; Lachman, O.; Dombrovsky, A. Aspects in tobamovirus management in modern agriculture: Cucumber green mottle mosaic virus. Acta Hortic. 2019, 1257, 1–8. [Google Scholar] [CrossRef]
- EPPO. Pest Risk Analysis for Tomato Brown Rugose Fruit Virus; European and Mediterranean Plant Protection Organization: Paris, France, 2020. [Google Scholar]
- Li, R.; Baysal-Gurel, F.; Abdo, Z.; Miller, S.A.; Ling, K.-S. Evaluation of disinfectants to prevent mechanical transmission of viruses and a viroid in greenhouse tomato production. Virol. J. 2015, 12, 5. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants-BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018. [Google Scholar]
- Lipman, D.J.; Pearson, W.R. Rapid and sensitive protein similarity searches. Science 1985, 227, 1435–1441. [Google Scholar] [CrossRef]
- Pearson, W.R.; Lipman, D.J. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 1988, 85, 2444–2448. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Ramakrishnan, C.; Sasisekharan, V. Stereochemistry of polypeptide chain configurations. J. Mol. Biol. 1963, 7, 95–99. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Schrödinger. Schrödinger Release 2021-3: LigPrep; Schrödinger, LLC.: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger. Schrödinger Release 2021-2: Glide; Schrödinger, LLC.: New York, NY, USA, 2021. [Google Scholar]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Statsoft Inc. Statistica for Windows (Data Analysis Software System), version 8.0.; Statsoft. Inc.: Tulsa, OK, USA, 2008. [Google Scholar]






| Treatments | Concentration (ppm) | Tukey HSD Test c | Inoculation | RT-PCR d | RT-PCR e | RT-PCR f | |||
|---|---|---|---|---|---|---|---|---|---|
| C S | S s | C S | S s | C S | S s | ||||
| 1 | 32 | a | YES | N | N | N | N | N | N |
| AME | 32 | a | YES | N | N | N | N | N | N |
| 2 | 32 | a | YES | N | N | N | N | N | N |
| NaOCl | 20,000 | a | YES | N | N | N | N | N | N |
| DW (PC) a | - | b | YES | P | P | P | P | P | P |
| DW (NC) b | - | a | NO | N | N | N | N | N | N |
| Treatments | Concentration (ppm) | Tukey HSD Test c | RT-PCR d | RT-PCR e | RT-PCR f |
|---|---|---|---|---|---|
| 1 | 32 | a | N | N | N |
| AME | 32 | a | N | N | N |
| 2 | 32 | a | N | N | N |
| NaOCl | 20,000 | a | N | N | N |
| DW (PC) a | - | b | P | P | P |
| DW (NC) b | - | a | N | N | N |
| Ligand Molecules | Glide Binding Energy (kcal/mol) | H-bond Interacting Amino Acids | Hydrophobic Interactions/π-Cation Interactions | Salt Bridge |
|---|---|---|---|---|
| 4-hydroxybenzoic acid | −25.688 | Asp219B, Glu222B, Lys253B, Pro254B, Lys268B | Asp219B, Lys253B, Val255B, Lys268B | Lys268 |
| Catechinic acid | −38.794 | Asn73A, Gly137A, Tyr139A, Ser143A, Lys268B | Val260B | - |
| Ribavirin | −35.285 | Asn73A, Lys134A, Gly137A, Leu138A, Ser143A, Pro254B, Lys268B | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iobbi, V.; Lanteri, A.P.; Minuto, A.; Santoro, V.; Ferrea, G.; Fossa, P.; Bisio, A. Autoxidation Products of the Methanolic Extract of the Leaves of Combretum micranthum Exert Antiviral Activity against Tomato Brown Rugose Fruit Virus (ToBRFV). Molecules 2022, 27, 760. https://doi.org/10.3390/molecules27030760
Iobbi V, Lanteri AP, Minuto A, Santoro V, Ferrea G, Fossa P, Bisio A. Autoxidation Products of the Methanolic Extract of the Leaves of Combretum micranthum Exert Antiviral Activity against Tomato Brown Rugose Fruit Virus (ToBRFV). Molecules. 2022; 27(3):760. https://doi.org/10.3390/molecules27030760
Chicago/Turabian StyleIobbi, Valeria, Anna Paola Lanteri, Andrea Minuto, Valentina Santoro, Giuseppe Ferrea, Paola Fossa, and Angela Bisio. 2022. "Autoxidation Products of the Methanolic Extract of the Leaves of Combretum micranthum Exert Antiviral Activity against Tomato Brown Rugose Fruit Virus (ToBRFV)" Molecules 27, no. 3: 760. https://doi.org/10.3390/molecules27030760