The Bright Side of the Tiger: Autofluorescence Patterns in Aedes albopictus (Diptera, Culicidae) Male and Female Mosquitoes
Abstract
:1. Introduction
2. Results
2.1. Autofluorescence in the Head Appendages of Male and Female Ae. albopictus Adults
2.2. Autofluorescence in Ae. albopictus Body Scales
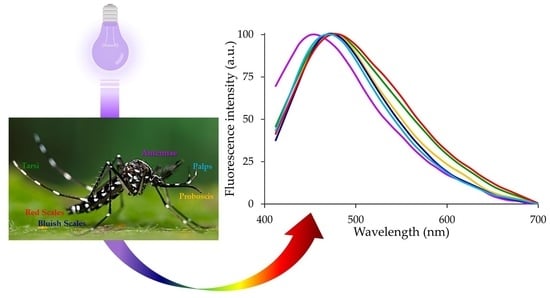
2.3. Spectrofluorometric Analysis
3. Discussion
4. Materials and Methods
4.1. Insect Samples
4.2. Bright Field and Fluorescence Microscopy
4.3. Spectrofluorimetric Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Udenfriend, S. Fluorescence Assay in Biology and Medicine, 1st ed.; Horecker, B., Kaplan, N.O., Marmur, J., Scheraga, H.A., Eds.; Academic Press: New York, NY, USA; London, UK, 1969; Volume 2, pp. 195–404. [Google Scholar]
- Croce, A.C. Light and autofluorescence, multitasking features in living organisms. Photochem 2021, 1, 67–125. [Google Scholar] [CrossRef]
- Lagorio, M.G.; Cordon, G.B.; Iriel, A. Reviewing the relevance of fluorescence in biological systems. Photochem. Photobiol. Sci. 2015, 14, 1538–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.; Johnsen, S. Fluorescence as a means of colour signal enhancement. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abels, J.P.; Ludescher, R.D. Native fluorescence from juvenile stages of common food storage insects. J. Agric. Food Chem. 2003, 51, 544–549. [Google Scholar] [CrossRef]
- Rosales, A.C.; Atencio, J.A.D.; Rodríguez, M.C.; Gutiérrez, E.G. In-vivo measurement of the fluorescence spectrum of wild cochineal (Dactylopius opuntiae). Sci. Rep. 2021, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Gebru, A.; Jansson, S.; Ignell, R.; Kirkeby, C.; Prangsma, J.C.; Brydegaard, M. Multiband modulation spectroscopy for the determination of sex and species of mosquitoes in flight. J. Biophotonics 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Cockayne, E.A.I. The distribution of fluorescent pigments in Lepidoptera. Trans. R. Entomol. Soc. Lond. 1924, 72, 1–19. [Google Scholar] [CrossRef]
- Wilts, B.D.; Trzeciak, T.M.; Vukusic, P.; Stavenga, D.G. Papiliochrome II pigment reduces the angle dependency of structural wing colouration in nireus group papilionids. J. Exp. Biol. 2012, 215, 796–805. [Google Scholar] [CrossRef] [Green Version]
- Wilts, B.D.; Ijbema, N.; Stavenga, D.G. Pigmentary and photonic coloration mechanisms reveal taxonomic relationships of the Cattlehearts (Lepidoptera: Papilionidae: Parides). BMC Evol. Biol. 2014, 14, 160. [Google Scholar] [CrossRef] [Green Version]
- Vigneron, J.P.; Kertész, K.; Vértesy, Z.; Rassart, M.; Lousse, V.; Bálint, Z.; Biró, L.P. Correlated diffraction and fluorescence in the backscattering iridescence of the male butterfly Troides magellanus (Papilionidae). Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2008, 78. [Google Scholar] [CrossRef] [Green Version]
- Mouchet, S.R.; Tabarrant, T.; Lucas, S.; Su, B.-L.; Vukusic, P.; Deparis, O. Vapor sensing with a natural photonic cell. Opt. Express 2016, 24, 12267. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Appel, E.; Gorb, S.N. Functional diversity of resilin in Arthropoda. Beilstein J. Nanotechnol. 2016, 7, 1241–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäumler, F.; Büsse, S. Resilin in the flight apparatus of Odonata (Insecta) cap tendons and their biomechanical importance for flight. Biol. Lett. 2019, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pentzold, S.; Marion-Poll, F.; Grabe, V.; Burse, A. Autofluorescence-based identification and functional validation of antennal gustatory sensilla in a specialist leaf beetle. Front. Physiol. 2019, 10, 343. [Google Scholar] [CrossRef]
- Saltin, B.D.; Matsumura, Y.; Reid, A.; Windmill, J.F.; Gorb, S.N.; Jackson, J.C. Resilin distribution and sexual dimorphism in the midge antenna and their influence on frequency sensitivity. Insects 2020, 11, 520. [Google Scholar] [CrossRef]
- Saltin, B.D.; Matsumura, Y.; Reid, A.; Windmill, J.F.; Gorb, S.N.; Jackson, J.C. Material stiffness variation in mosquito antennae. J. R. Soc. Interface 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- Wong, P.S.J.; Li, M.; Zhi, I.; Chong, C.S.; Ng, L.C.; Tan, C.H. Aedes (Stegomyia) albopictus (Skuse): A potential vector of Zika virus in Singapore. PLoS Negl. Trop. Dis. 2013, 7, e2348. [Google Scholar] [CrossRef]
- Vega-Rúa, A.; Marconcini, M.; Madec, Y.; Manni, M.; Carraretto, D.; Gomulski, L.M.; Gasperi, G.; Failloux, A.B.; Malacrida, A.R. Vector competence of Aedes albopictus populations for Chikungunya virus is shaped by their demographic history. Commun. Biol. 2020, 3, 1–13. [Google Scholar] [CrossRef]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R.; Failloux, A.B. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika virus in Gabon (Central Africa) 2007: A new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jupille, H.; Seixas, G.; Mousson, L.; Sousa, C.A.; Failloux, A.B. Zika virus, a new threat for Europe? PLoS Negl. Trop. Dis. 2016, 10, e0004901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, P.M.; Ehrlich, H.Y.; Magalhaes, T.; Miller, M.R.; Conway, P.J.; Bransfield, A.; Misencik, M.J.; Gloria-Soria, A.; Warren, J.L.; Andreadis, T.G.; et al. Successive blood meals enhance virus dissemination within mosquitoes and increase transmission potential. Nat. Microbiol. 2020, 5, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Wilke, A.B.B.; Benelli, G.; Beier, J.C. Beyond frontiers: On invasive alien mosquito species in America and Europe. PLoS Negl. Trop. Dis. 2020, 14, e0007864. [Google Scholar] [CrossRef] [PubMed]
- Nansen, C. Penetration and scattering two optical phenomena to consider when applying proximal remote sensing technologies to object classifications. PLoS ONE 2019, 13, e0204579. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. Blood, sex, and the mosquito. BioScience 1995, 45, 326–331. [Google Scholar] [CrossRef]
- Rueda, L.M. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with Dengue virus transmission. Zootaxa 2004, 589, 1–60. [Google Scholar] [CrossRef]
- Huang, Y.-M. Neotype designation for Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae). Proc. Entomol. Soc. Wash 1968, 70, 297–302. [Google Scholar]
- McIver, S. Comparative studies on the sense organs on the antennae and maxillary palps of selected male culicine mosquitoes. Can. J. Zool. 1971, 49, 235–239. [Google Scholar] [CrossRef]
- Hawley, W.A. The biology of Aedes albopictus. J. Am. Mosq. Control. Assoc. Suppl. 1988, 1, 1–39. [Google Scholar]
- Cerkvenik, U.; Dodou, D.; van Leeuwen, J.L.; Gussekloo, S.W.S. Functional principles of steerable multi-element probes in insects. Biol. Rev. Camb. Philos. Soc. 2019, 94, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.; Merzendorfer, H.; Arakane, Y.; Kramer, K.J. Chitin metabolism in insects. Insect Mol. Biol. Biochem. 2012, 193–235. [Google Scholar]
- Neff, D.; Frazier, S.F.; Quimby, L.; Wang, R.T.; Zill, S. Identification of resilin in the leg of cockroach, Periplaneta americana: Confirmation by a simple method using pH dependence of UV fluorescence. Arthropod Struct. Dev. 2000, 29, 75–83. [Google Scholar] [CrossRef]
- Gorb, S.N. Design of insect unguitractor apparatus. J. Morphol. 1996, 230, 219–230. [Google Scholar] [CrossRef]
- Chang, M.P.; Huang, W.; Mai, D.J. Monomer-scale design of functional protein polymers using consensus repeat sequences. J. Polym. Sci. 2021, 59, 2644–2664. [Google Scholar] [CrossRef]
- Andersen, S. Structure and function of resilin. In Elastomeric Proteins: Structures, Biomechanical Properties, and Biological Roles; Shewry, P., Tatham, A., Bailey, A., Eds.; Cambridge University Press: Cambridge, UK, 2003; pp. 259–278. [Google Scholar]
- Gorb, S.N. Serial elastic elements in the damselfly wing: Mobile vein joints contain resilin. Naturwissenschaften 1999, 86, 552–555. [Google Scholar] [CrossRef]
- He, N.; Botelho, J.M.C.; McNall, R.J.; Belozerov, V.; Dunn, W.A.; Mize, T.; Orlando, R.; Willis, J.H. Proteomic analysis of cast cuticles from Anopheles gambiae by tandem mass spectrometry. Insect Biochem. Mol. Biol. 2007, 37, 135–146. [Google Scholar] [CrossRef]
- Ramasamy, R.; Thiruchenthooran, V.; Jayadas, T.T.P.; Eswaramohan, T.; Santhirasegaram, S.; Sivabalakrishnan, K.; Naguleswaran, A.; Uzest, M.; Cayrol, B.; Voisin, S.N.; et al. Transcriptomic, proteomic and ultrastructural studies on salinity-tolerant Aedes aegypti in the context of rising sea levels and arboviral disease epidemiology. BMC Genom. 2021, 22, 253. [Google Scholar] [CrossRef]
- Curtin, T.J.; Jones, J.C. The mechanism of ovulation and oviposition in Aedes aegypti. Ann. Entomol. Soc. Am. 1961, 54, 298–313. [Google Scholar] [CrossRef]
- Clements, A.N.; Potter, S.A. The fine structure of the spermathecae and their ducts in the mosquito Aedes aegypti. J. Insect Physiol. 1967, 13, 1825–1836. [Google Scholar] [CrossRef]
- Pascini, T.V.; Ramalho-Ortigão, M.; Martins, G.F. Morphological and morphometrical assessment of spermathecae of Aedes aegypti females. Mem. Inst. Oswaldo Cruz 2012, 107, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Giglioli, M.E. The female reproductive system of Anopheles gambiae melas. The structure and function of the genital ducts and associated organs. Riv. Malariol. 1963, 42, 149–176. [Google Scholar]
- Lee, R.M.K.W.; Craig, D.A. The labrum and labral sensilla of mosquitoes (Diptera: Culicidae): A scanning electron microscope study. Can. J. Zool. 1983, 61, 1568–1579. [Google Scholar] [CrossRef]
- Scolari, F.; IGM-CNR, Pavia, Italy. Personal Communication, 2021.
- Nuttall, G.H.F.; Shipley, A.E. Studies in relation to malaria: II. The structure and biology of Anopheles. J. Hyg. 1901, 1, 451–484. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Bulut, E.; Mujtaba, M.; Sivickis, K.; Sargin, I.; Akyuz, B.; Erdogan, S. Gender influences differentiation of chitin among body parts. Arch. Insect Biochem. Physiol. 2016, 93, 96–109. [Google Scholar] [CrossRef]
- Tauber, O.E. The distribution of chitin in an insect. J. Morphol. 1934, 56, 51–58. [Google Scholar] [CrossRef]
- Qin, G.; Hu, X.; Cebe, P.; Kaplan, D.L. Mechanism of resilin elasticity. Nat. Commun. 2012, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Lapidot, S.; Numata, K.; Hu, X.; Meirovitch, S.; Dekel, M.; Podoler, I.; Shoseyov, O.; Kaplan, D.L. Expression, cross-linking, and characterization of recombinant chitin binding resilin. Biomacromolecules 2009, 10, 3227–3234. [Google Scholar] [CrossRef]
- Appel, E.; Heepe, L.; Lin, C.P.; Gorb, S.N. Ultrastructure of dragonfly wing veins: Composite structure of fibrous material supplemented by resilin. J. Anat. 2015, 227, 561–582. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Aydemir, B.E.; Dumanli, A.G. Understanding the structural diversity of chitins as a versatile biomaterial. Philos. Trans. A Math. Phys. Eng. Sci. 2021, 379, 1–19. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Zhu, K.Y. Chitin in arthropods: Biosynthesis, modification, and metabolism. Adv. Exp. Med. Biol. 2019, 1142, 169–207. [Google Scholar] [PubMed]
- Rabasović, M.D.; Pantelić, D.V.; Jelenković, B.M.; Ćurčić, S.B.; Rabasović, M.S.; Vrbica, M.D.; Lazović, V.M.; Ćurčić, B.P.M.; Krmpot, A.J. Nonlinear microscopy of chitin and chitinous structures: A case study of two cave-dwelling insects. J. Biomed. Opt. 2015, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Gorb, S.N. Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J. Microsc. 2012, 245, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Peisker, H.; Michels, J.; Gorb, S.N. Evidence for a material gradient in the adhesive tarsal setae of the ladybird beetle Coccinella septempunctata. Nat. Commun. 2013, 4, 1661. [Google Scholar] [CrossRef] [Green Version]
- Göpfert, M.C.; Briegel, H.; Robert, D. Mosquito hearing: Sound-induced antennal vibrations in male and female Aedes aegypti. J. Exp. Biol. 1999, 202, 2727–2738. [Google Scholar] [CrossRef]
- Robert, D. Insect bioacoustics: Mosquitoes make an effort to listen to each other. Curr. Biol. 2009, 19, R446–R449. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.; Russell, I. Flying in tune: Sexual recognition in mosquitoes. Curr. Biol. 2006, 16, 1311–1316. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Qiu, Y.T.; Wang, G.; Kwon, J.Y.; Rutzler, M.; Kwon, H.W.; Pitts, R.J.; van Loon, J.J.A.; Takken, W.; Carlson, J.R.; et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 2007, 17, 1533–1544. [Google Scholar] [CrossRef] [Green Version]
- McIver, S.; Charlton, C. Studies on the sense organs on the palps of selected culicine mosquitoes. Can. J. Zool. 1970, 48, 293–295. [Google Scholar] [CrossRef]
- Bohbot, J.D.; Sparks, J.T.; Dickens, J.C. The maxillary palp of Aedes aegypti, a model of multisensory integration. Insect Biochem. Mol. Biol. 2014, 48, 29–39. [Google Scholar] [CrossRef]
- Grant, A.J.; Wigton, B.E.; Aghajanian, J.G.; O’Connell, R.J. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J. Comp. Physiol. A 1995, 177, 389e396. [Google Scholar] [CrossRef] [PubMed]
- Thom, C.; Guerenstein, P.G.; Mechaber, W.L.; Hildebrand, J.G. Floral CO2 reveals flower profitability to moths. J. Chem. Ecol. 2004, 30, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Athrey, G.; Popkin-Hall, Z.R.; Takken, W.; Slotman, M.A. The expression of chemosensory genes in male maxillary palps of Anopheles coluzzii (Diptera: Culicidae) and An. quadriannulatus. J. Med. Entomol. 2021, 58, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shang, Y.; Hilton, D.S.; Inthavong, K.; Zhang, D.; Elgar, M.A. Antennal scales improve signal detection efficiency in moths. Proc. Biol. Sci. 2018, 285, 20172832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clements, A.N. The Biology of Mosquitoes: Development, Nutrition and Reproduction; Chapman & Hall: London, UK, 1992; Volume 1, p. 532. [Google Scholar]
- Neil, T.R.; Shen, Z.; Robert, D.; Drinkwater, B.W.; Holderied, M.W. Moth wings are acoustic metamaterials. Proc. Natl. Acad. Sci. USA 2020, 117, 31134–31141. [Google Scholar] [CrossRef]
- Ito, K.; Yoshikawa, M.; Fujii, T.; Tabunoki, H.; Yokoyama, T. Melanin pigmentation gives rise to black spots on the wings of the silkworm Bombyx mori. J. Insect Physiol. 2016, 91–92, 100–106. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Monteiro, A. Melanin pathway genes regulate color and morphology of butterfly wing scales. Cell Rep. 2018, 24, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Whitten, M.M.A.; Coates, C.J. Re-evaluation of insect melanogenesis research: Views from the dark side. Pigment. Cell Melanoma Res. 2017, 30, 386–401. [Google Scholar] [CrossRef]
- Kuwalekar, M.; Deshmukh, R.; Padvi, A.; Kunte, K. Molecular evolution and developmental expression of melanin pathway genes in Lepidoptera. Front. Ecol. Evol. 2020, 8, 226. [Google Scholar] [CrossRef]
- Mostert, A.B. Melanin, the what, the why and the how: An introductory review for materials scientists interested in flexible and versatile polymers. Polymers 2021, 13, 1670. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Leertouwer, H.L.; Hariyama, T.; de Raedt, H.A.; Wilts, B.D. Sexual dichromatism of the damselfly Calopteryx japonica caused by a melanin-chitin multilayer in the male wing veins. PLoS ONE 2012, 7, e49743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Meredith, P.; Powell, B.J.; Riesz, J.; Nighswander-Rempel, S.P.; Pederson, M.R.; Moore, E.G. Towards structure-property-function relationships for eumelanin. Soft Matter. 2006, 2, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Nahhas, A.; Pascher, T.; Leone, L.; Panzella, L.; Napolitano, A.; Sundström, V. Photochemistry of pheomelanin building blocks and model chromophores: Excited-state intra- and intermolecular proton transfer. J. Phys. Chem. Lett. 2014, 5, 2094–2100. [Google Scholar] [CrossRef]
- Djokic, S.; Bakhrat, A.; Tsurim, I.; Urakova, N.; Rasgon, J.L.; Abdu, U. Actin bundles play a different role in shaping scales compared to bristles in the mosquito Aedes aegypti. Sci. Rep. 2020, 10, 14885. [Google Scholar] [CrossRef]
- Winterton, S.L. Chapter 229 Scales and Setae. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press (Elsevier): Burlington, MA, USA, 2009; pp. 901–904. [Google Scholar]
- Tsuda, Y.; Yotopranoto, S.; Bendryman, S.S.; Rosmanida Dachlan, Y.P.; Takagi, M. Seasonal changes in variation of dorsal scale pattern of Aedes aegypti (L.) (Diptera: Culicidae) in Surabaya, Indonesia. Med. Entomol. Zool. 2003, 54, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Theobald, F.V. Genera insectorum, Diptera. Fam. Culicidae; L. Desmet-Verteneuil: Brussels, Belgium, 1905; Volume 26, pp. 1–50. [Google Scholar]
- Theobald, F.V. The classification of mosquitoes. J. Trop. Med. 1901, 4, 229–235. [Google Scholar]
- Ong, S.Q.; Ahmad, H.; Nair, G.; Isawasan, P.; Majid, A.H.A. Implementation of a deep learning model for automated classification of Aedes aegypti (Linnaeus) and Aedes slbopictus (Skuse) in Real Time. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Verna, T.N.; Munstermann, L.E. Morphological variants of Aedes aegypti collected from the Leeward Island of Antigua. J. Am. Mosq. Control Assoc. 2011, 27, 308–311. [Google Scholar] [CrossRef] [Green Version]









| Structure | Peak Maximum * | FWHM * |
|---|---|---|
| Labella | 470–480 nm | 140–145 nm |
| Maxillary palps | 460–480 nm | 120–130 nm |
| Antennal elements | ||
| Male flagellomer/female blue disc Female greenish cone | 440–470 nm 460–490 nm | 123–130 nm 132–140 nm |
| Abdominal scales | ||
| Bluish Reddish | 460–490 nm 470–500 nm | 137–145 nm 147–160 nm |
| Hindleg tarsi | 470–480 nm | 153–157 nm |
| Fore trochanter–femur joint | 430–455 nm | 134 nm |
| Maxilla | 465–490 nm | 155 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croce, A.C.; Scolari, F. The Bright Side of the Tiger: Autofluorescence Patterns in Aedes albopictus (Diptera, Culicidae) Male and Female Mosquitoes. Molecules 2022, 27, 713. https://doi.org/10.3390/molecules27030713
Croce AC, Scolari F. The Bright Side of the Tiger: Autofluorescence Patterns in Aedes albopictus (Diptera, Culicidae) Male and Female Mosquitoes. Molecules. 2022; 27(3):713. https://doi.org/10.3390/molecules27030713
Chicago/Turabian StyleCroce, Anna C., and Francesca Scolari. 2022. "The Bright Side of the Tiger: Autofluorescence Patterns in Aedes albopictus (Diptera, Culicidae) Male and Female Mosquitoes" Molecules 27, no. 3: 713. https://doi.org/10.3390/molecules27030713