Cytotoxic 13,28 Epoxy Bridged Oleanane-Type Triterpenoid Saponins from the Roots of Ardisia crispa
Abstract
:1. Introduction
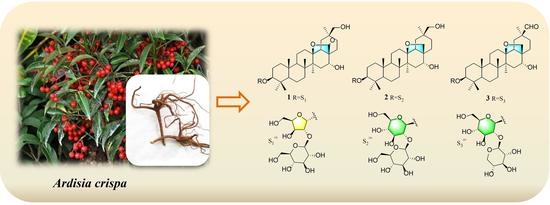
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization of Compounds 1–3
3.5. Acid Hydrolysis of Ardisiacrispin D-F (1–3)
3.6. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kobayashi, H.; Mejía, E. The genus Ardisia: A novel source of health-promoting compounds and phytopharmaceuticals. J. Ethnopharmacol. 2005, 96, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Li, W.; Jia, Z.; Satou, T.; Fushiya, S.; Koike, K. Biologically active triterpenoid saponins from Ardisia japonica. J. Nat. Prod. 2007, 70, 179–187. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, H.F.; Qiu, F.; Wang, X.J.; Chen, X.L.; Wen, A.D. Triterpenoid saponins from Ardisia pusilla and their cytotoxic activity. Planta Med. 2009, 75, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.H.; Gong, Q.Q.; Zhao, H.X.; Liu, P. Triterpenoid saponins from Ardisia gigantifolia. Chem. Pharm. Bull. 2010, 58, 1248–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, L.H.; Wei, N.Y.; Liu, P. Cytotoxic triterpenoid saponins from Ardisia gigantifolia. Planta Med. 2012, 78, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.F.; Xu, J.F.; Feng, Z.M.; Zhang, P.C. Cytotoxic triterpenoid saponins from the roots of Ardisia crenata. J. Asian Nat. Prod. Res. 2008, 10, 833–839. [Google Scholar] [CrossRef]
- Gong, Q.Q.; Mu, L.H.; Liu, P.; Yang, S.L.; Wang, B.; Feng, Y.L. New triterpenoid sapoin from Ardisia gigantifolia Stapf. Chin. Chem. Lett. 2010, 21, 449–452. [Google Scholar] [CrossRef]
- Tang, H.F.; Yun, J.; Lin, H.W.; Chen, X.L.; Wang, X.J.; Cheng, G. Two new triterpenoid saponins cytotoxic to human glioblastoma U251MG cells from Ardisia pusilla. Chem. Biodivers. 2009, 6, 1443–1452. [Google Scholar] [CrossRef]
- Editorial Committee of Nan Jing University of Chinese Medicine, Chinese Materia Medica. Zhong Yao Da Ci Dian; Shanghai Science and Technology Press: Shanghai, China, 2014; Volume 1, p. 1181. [Google Scholar]
- Nordin, M.L.; Kadir, A.A.; Zakaria, Z.A.; Abdullah, R.; Abdullah, M. In vitro investigation of cytotoxic and antioxidative activities of Ardisia crispa against breast cancer cell lines, MCF-7 and MDA-MB-231. BMC Complement. Altern. Med. 2018, 18, 87. [Google Scholar] [CrossRef] [Green Version]
- Yeong, L.T.; Hamid, R.A.; Yazan, L.S.; Khaza’ai, H.; Mohtarrudin, N. Low dose triterpene-quinone fraction from Ardisia crispa root precludes chemical-induced mouse skin tumor promotion. BMC Complement. Altern. Med. 2015, 15, 431. [Google Scholar] [CrossRef] [Green Version]
- Jun, L.W.; Foong, C.P.; Hamid, R.A. Ardisia crispa root hexane fraction suppressed angiogenesis in human umbilical vein endothelial cells (HUVECs) and in vivo zebrafish embryo model. Biomed. Pharmacother. 2019, 118, 109221. [Google Scholar] [CrossRef] [PubMed]
- Yeong, L.T.; Hamid, R.A.; Yazan, L.S.; Khaza’ai, H.; Hamsin, D. Synergistic action of compounds isolated from the hexane extract of Ardisia crispa root against tumour-promoting effect, in vitro. Nat. Prod. Res. 2014, 28, 2026–2030. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.A.; Othman, F.; Anthony, J.J.; Ting, Y.L. Chemopreventive effect of Ardisia crispa hexane fraction on the peri-initiation phase of mouse skin tumorigenesis. Med. Princ. Pract. 2013, 22, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Nordin, M.L.; Kadir, A.A.; Zakaria, Z.A.; Othman, F.; Abdullah, R.; Abdullah, M. Cytotoxicity and apoptosis induction of Ardisia crispa and its solvent partitions against Mus musculus mammary carcinoma cell line (4T1). Evid-Based Complement. Altern. Med. 2017, 2017, 9368079. [Google Scholar] [CrossRef]
- Hamsin, D.; Hamid, R.A.; Yazan, L.S.; Taib, C.N.; Yeong, L.T. Ardisia crispa roots inhibit cyclooxygenase and suppress angiogenesis. BMC Complement. Altern. Med. 2014, 14, 102. [Google Scholar]
- Hamsin, D.; Hamid, R.A.; Yazan, L.S.; Taib, C.N.; Yeong, L.T. The hexane fraction of Ardisia crispa Thunb. A. DC. roots inhibits inflammation-induced angiogenesis. BMC Complement. Altern. Med. 2013, 13, 5. [Google Scholar] [CrossRef]
- Sulaiman, H.; Hamid, R.A.; Yeong, L.T.; Othman, F. Anti-tumor effect of Ardisia crispa hexane fraction on 7, 12-dimethylbenz[α]anthracene-induced mouse skin papillomagenesis. J. Cancer Res. Ther. 2012, 8, 404–410. [Google Scholar]
- Jia, Z.; Koike, K.; Ohmoto, T.; Ni, M. Triterpenoid saponins from Ardisia crenata. Phytochemistry 1994, 37, 1389–1396. [Google Scholar] [CrossRef]
- Li, S.N.; Sun, J.F.; Wang, J.M.; Jin, L.; Zong, T.Q.; Zhou, W.; Li, G. Two new phenolic glycosides from the fruits of Illicium verum. J. Asian Nat. Prod. Res. 2021, 24, 31–38. [Google Scholar] [CrossRef]
- Yamasaki, K.; Tanaka, O. Saponins from Vietnamese ginseng, Panax vietnamensis HA et Grushv. Collected in central Vietnam. II. Chem. Pharm. Bull. 1994, 42, 115–122. [Google Scholar]
- Lavaud, C.; Massiot, G.; Moretti, C.; Men-Olivier, L.L. Triterpene saponins from Myrsine pellucida. Phytochemistry 1994, 37, 1671–1677. [Google Scholar] [CrossRef]
- Mu, L.H.; Zhang, J.; Liu, P. Biotransformation and antitumor activity of triterpenoid derivatives from Ardisia gigantifolia. Chin. Tradit. Herb. Drugs 2018, 49, 1266–1271. [Google Scholar]
- Huang, J.; Zhang, H.; Shimizu, N.; Takeda, T. Ardisimamillosides G and H, two new triterpenoid saponins from Ardisia mamillata. Chem. Pharm. Bull. 2003, 51, 875–877. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Liu, Y.; Pan, J.; Ye, H.L.; Sun, Y.; Zhao, D.Y.; Kuang, H.X.; Yang, B.Y. Melongenaterpenes A-L, Vetispirane-Type Sesquiterpenoids from the Roots of Solanum melongena. J. Nat. Prod. 2019, 82, 3242–3248. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Yin, X.; Liu, Y.; Zhao, D.Y.; Kuang, H.X. New steroidal saponins from the roots of Solanum melongena L. Fitoterapia 2018, 128, 12–19. [Google Scholar] [CrossRef]
- Yang, B.Y.; Yin, X.; Liu, Y.; Sun, Y.; Guan, W.; Zhou, Y.Y.; Kuang, H.X. Terpenes and lignans from the roots of Solanum melongena L. Nat. Prod. Res. 2020, 34, 359–368. [Google Scholar] [CrossRef]



| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| Position | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) |
| 1 | 40.0 | 1.78 (m) 0.98 (m) | 40.2 | 1.73 (m) 1.00 (m) | 40.2 | 1.73 (m) 0.97 (m) |
| 2 | 26.7 | 1.96 (m) 1.67 (m) | 27.3 | 1.95 (m) 1.70 (m) | 27.3 | 1.85 (m) 1.70 (m) |
| 3 | 89.4 | 3.15 (overlap) | 91.3 | 3.16 (overlap) | 91.3 | 3.14 (dd, 11.3, 4.2) |
| 4 | 40.0 | - | 40.5 | - | 40.6 | - |
| 5 | 56.5 | 0.79 (brd, 7.1) | 56.8 | 0.72 (brd, 11.1) | 56.8 | 0.72 (brd, 9.7) |
| 6 | 18.8 | 1.51 (m) 1.46 (m) | 18.7 | 1.51 (m) 1.42 (m) | 18.7 | 1.49 (m) 1.42 (m) |
| 7 | 34.8 | 1.59 (m) 1.24 (m) | 35.1 | 1.56 (m) 1.20 (m) | 35.1 | 1.54 (m) 1.20 (m) |
| 8 | 43.1 | - | 43.3 | - | 43.4 | - |
| 9 | 50.8 | 1.35 (overlap) | 51.6 | 1.46 (overlap) | 51.3 | 1.24 (overlap) |
| 10 | 37.8 | - | 37.7 | - | 37.8 | - |
| 11 | 19.4 | 1.65 (m) 1.50 (m) | 19.8 | 1.64 (m) 1.48 (m) | 19.8 | 1.63 (m) 1.48 (m) |
| 12 | 32.1 | 2.16 (m) 1.56 (m) | 32.8 | 2.05 (m) 1.33 (m) | 33.2 | 2.10 (m) 1.26 (m) |
| 13 | 95.0 | - | 88.5 | - | 88.2 | - |
| 14 | 43.0 | - | 45.3 | - | 45.3 | - |
| 15 | 37.4 | 1.78 (overlap) 1.44 (overlap) | 36.9 | 2.08 (overlap) 1.20 (overlap) | 37.0 | 2.07 (m) 1.20 (m) |
| 16 | 73.3 | 3.96 (brd, 5.2) | 78.0 | 3.89 (overlap) | 77.8 | 3.91 (brd, 4.9) |
| 17 | 49.5 | - | 45.2 | - | 44.8 | - |
| 18 | 51.9 | 1.85 (m) | 51.3 | 1.24 (m) | 54.0 | 1.12 (dd, 12.9, 2.1) |
| 19 | 33.1 | 2.35 (t, like, 12.4) 1.68 (m) | 33.8 | 2.25 (t, like, 12.6) 1.58 (m) | 34.0 | 2.50 (dd, 14.2, 12.9) 1.96 (dd, 14.2, 2.1) |
| 20 | 36.7 | - | 36.9 | - | 49.2 | - |
| 21 | 32.3 | 2.03 (m) 1.35 (m) | 33.2 | 2.04 (m) 1.28 (m) | 30.8 | 2.12 (m) 1.89 (m) |
| 22 | 28.6 | 1.84 (m) 1.60 (m) | 31.8 | 1.72 (m) 1.45 (m) | 32.8 | 1.84 (m) 1.32 (m) |
| 23 | 28.7 | 0.99 (s) | 28.3 | 1.06 (s) | 28.3 | 1.05 (s) |
| 24 | 16.8 | 0.78 (s) | 16.7 | 0.83 (s) | 16.7 | 0.83 (s) |
| 25 | 16.7 | 0.91 (s) | 16.7 | 0.89 (s) | 16.7 | 0.89 (s) |
| 26 | 18.2 | 1.08 (s) | 18.8 | 1.14 (s) | 18.8 | 1.13 (s) |
| 27 | 19.8 | 1.40 (s) | 20.0 | 1.25 (s) | 20.1 | 1.27 (s) |
| 28 | 181.0 | - | 78.6 | 3.50 (d, 7.6) 3.10 (d, 7.6) | 78.4 | 3.48 (d, 7.6) 2.98 (d, 7.6) |
| 29 | 28.3 | 0.92 (s) | 28.5 | 0.92 (s) | 24.3 | 0.97 (s) |
| 30 | 66.5 | 3.55 (d, 11.1) 3.25 (d, 11.1) | 66.5 | 3.55 (d, 10.9) 3.30 (d, 10.9) | 209.2 | 9.40 (s) |
| 1′ | 110.2 | 5.23 (brs) | 105.9 | 4.39(d, 7.8) | 106.0 | 4.40 (d, 7.0) |
| 2′ | 89.6 | 4.21 (d, 5.2) | 79.1 | 3.87 (m) | 80.9 | 3.56 (m) |
| 3′ | 75.8 | 4.26 (dd, 5.2, 2.0) | 76.3 | 3.20 (m) | 78.0 | 3.53 (m) |
| 4′ | 83.9 | 4.19 (m) | 70.3 | 3.83 (m) | 71.1 | 3.49 (m) |
| 5′ | 62.5 | 3.83 (dd, 11.6, 4.9) 3.75 (dd, 11.6, 6.4) | 76.2 | 3.47 (m) | 78.4 | 3.47 (m) |
| 6′ | - | - | 62.3 | 3.72 (overlap) 3.70 (overlap) | 63.0 | 3.81 (dd, 11.9, 2.0) 3.60 (dd, 11.9, 5.8) |
| 1″ | 104.2 | 4.44 (d, 7.8) | 104.6 | 4.65 (d, 7.7) | 104.6 | 4.65 (d, 7.7) |
| 2″ | 75.0 | 3.18 (m) | 75.4 | 3.66 (m) | 76.2 | 3.20 (m) |
| 3″ | 78.0 | 3.25 (m) | 78.2 | 3.22 (m) | 77.9 | 3.33 (m) |
| 4″ | 71.3 | 3.36 (m) | 71.9 | 3.21 (m) | 71.9 | 3.21 (m) |
| 5″ | 77.9 | 3.34 (m) | 77.9 | 3.34 (m) | 66.5 | 3.84 (overlap) 3.17 (overlap) |
| 6″ | 62.4 | 3.85 (dd, 12.0, 2.5) 3.72 (dd, 12.0, 5.0) | 63.1 | 3.81 (dd, 11.9, 2.2) 3.61 (dd, 11.9, 5.9) | - | - |
| Compounds | IC50 (Mean ± SD μM) | ||
|---|---|---|---|
| HeLa | HepG2 | U87 MG | |
| 1 | 3.2 ± 0.7 | 8.8 ± 2.2 | 5.7 ± 1.8 |
| 2 | 4.4 ± 1.1 | 21.9 ± 6.1 | 6.8 ± 2.2 |
| 3 | 2.2 ± 0.6 | 33.6 ± 6.8 | 7.7 ± 0.9 |
| 4 | 6.8 ± 1.2 | 7.9 ± 2.2 | 6.6 ± 1.6 |
| 5 | 9.5 ± 1.8 | 14.4 ± 2.1 | 2.3 ± 0.4 |
| 6 | 5.4 ± 0.9 | 8.7 ± 1.2 | 6.8 ± 1.1 |
| 7 | 5.2 ± 1.3 | 38.2 ± 6.6 | 5.4 ± 0.9 |
| Cisplatin a | 9.8 ± 0.4 | 12.1 ± 0.3 | 16.7± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Hu, R.; Zhou, Y.; Zhu, W.; Zhou, Y. Cytotoxic 13,28 Epoxy Bridged Oleanane-Type Triterpenoid Saponins from the Roots of Ardisia crispa. Molecules 2022, 27, 1061. https://doi.org/10.3390/molecules27031061
Yin X, Hu R, Zhou Y, Zhu W, Zhou Y. Cytotoxic 13,28 Epoxy Bridged Oleanane-Type Triterpenoid Saponins from the Roots of Ardisia crispa. Molecules. 2022; 27(3):1061. https://doi.org/10.3390/molecules27031061
Chicago/Turabian StyleYin, Xin, Ruihang Hu, Yongqiang Zhou, Weiqian Zhu, and Ying Zhou. 2022. "Cytotoxic 13,28 Epoxy Bridged Oleanane-Type Triterpenoid Saponins from the Roots of Ardisia crispa" Molecules 27, no. 3: 1061. https://doi.org/10.3390/molecules27031061