The Influence of Lactic Acid Fermentation on Selected Properties of Pickled Red, Yellow, and Green Bell Peppers
Abstract
:1. Introduction
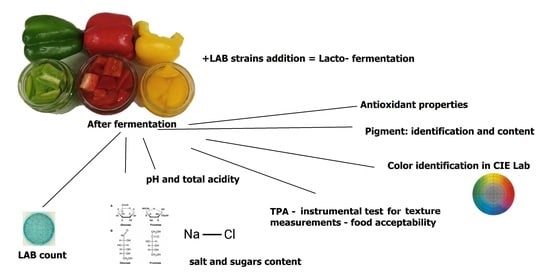
2. Results and Discussion
2.1. Determination of Basic Properties of Fermented Bell Peppers
2.2. Color Coefficients
2.3. Salt Content
2.4. Bacteria Count
2.5. Texture Analysis
2.6. Pigment Amount and Identification
2.7. Antioxidant Properties
3. Materials and Methods
3.1. Materials
3.2. Technological Treatment
Fermentation Process
3.3. Analytical Method
3.3.1. Dry Matter Content
3.3.2. Color Parameters
3.3.3. Texture Analysis
3.3.4. Vitamin C
3.3.5. Sugar Content
3.3.6. SEM-EDS Analysis
3.3.7. Pigment Content
3.3.8. Total Phenolic Content and Antioxidant Activity
3.3.9. Determination of the Number of Lactic Acid Bacteria
3.4. Statistical Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niakousari, M.; Razmjooei, M.; Nejadmansouri, M.; Barba, F.J.; Marszałek, K.; Koubaa, M. Current Developments in Industrial Fermentation Processes. In Fermentation Processes: Emerging and Conventional Technologies; Koubaa, M., Barba, F.J., Roohinejad, S., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 23–96. [Google Scholar]
- Jackson, R.S. Chapter 7—Fermentation. In Wine Science, 5th ed.; Jackson, R.S., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 461–572. [Google Scholar]
- Mustafa, S.M.; Chua, L.S. 13—Green Technological Fermentation for Probioticated Beverages for Health Enhancement. In Biotechnological Progress and Beverage Consumption; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 407–434. [Google Scholar]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds from food and by-products. Curr. Opin. Food Sci. 2022, 43, 189–198. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Wiktor, A.; Szczepańska, J.; Skąpska, S.; Witrowa-Rajchert, D.; Saraiva, J.A.; Lorenzo, J.M.; Barba, F.J. Emerging Technologies and Their Mechanism of Action on Fermentation. In Fermentation Processes: Emerging and Conventional Technologies; Koubaa, M., Barba, F.J., Roohinejad, S., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 117–144. [Google Scholar]
- Garcia, C.; Remize, F. Lactic acid fermentation of fruit and vegetable juices and smoothies: Innovation and health aspects. In Lactic Acid Bacteria in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 27–46. [Google Scholar]
- Kiczorowski, P.; Kiczorowska, B.; Samolińska, W.; Szmigielski, M.; Winiarska-Mieczan, A. Effect of fermentation of chosen vegetables on the nutrient, mineral, and biocomponent profile in human and animal nutrition. Sci. Rep. 2022, 12, 13422. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ten, M.M.Z.; Zwe, Y.H.; Li, D. Lactiplantibacillus plantarum 299v as starter culture suppresses Enterobacteriaceae more efficiently than spontaneous fermentation of carrots. Food Microbiol. 2022, 103, 103952. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Paulino do Nascimento, L.C.; Lacerda, D.C.; Ferreira, D.J.S.; de Souza, E.L.; de Brito Alves, J.L. Limosilactobacillus fermentum, Current Evidence on the Antioxidant Properties and Opportunities to be Exploited as a Probiotic Microorganism. Probiotics Antimicrob. Proteins 2022, 14, 960–979. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Mora, Z.V.-d.l.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell peppers (Capsicum annum L.) losses and wastes: Source for food and pharmaceutical applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: Regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2022, 19, 1–27. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Barroca, M.J. Effect of Drying on the Textural Attributes of Bell Pepper and Pumpkin. Dry. Technol. 2011, 29, 1911–1919. [Google Scholar] [CrossRef]
- Hu, X.; Saravanakumar, K.; Jin, T.; Wang, M.H. Effects of yellow and red bell pepper (paprika) extracts on pathogenic microorganisms, cancerous cells and inhibition of survivin. J. Food Sci. Technol. 2021, 58, 1499–1510. [Google Scholar] [CrossRef]
- Hallmann, E.; Marszałek, K.; Lipowski, J.; Jasińska, U.; Kazimierczak, R.; Średnicka-Tober, D.; Rembiałkowska, E. Polyphenols and carotenoids in pickled bell pepper from organic and conventional production. Food Chem. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Ríos, A.K.; Medina-Juárez, L.Á.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of the phenolic and oily fractions of different sweet bell peppers. J. Mex. Chem. Soc. 2013, 57, 137–143. [Google Scholar] [CrossRef]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive compounds, antioxidant activity and inhibition of key enzymes relevant to Alzheimer’s disease from sweet pepper (Capsicum annuum) extracts. Prev. Nutr. Food Sci. 2019, 24, 327. [Google Scholar] [CrossRef] [PubMed]
- Ropelewska, E.; Sabanci, K.; Aslan, M.F. The Changes in Bell Pepper Flesh as a Result of Lacto-Fermentation Evaluated Using Image Features and Machine Learning. Foods 2022, 11, 2956. [Google Scholar] [CrossRef] [PubMed]
- Althaus, B.; Blanke, M. Non-destructive, opto-electronic determination of the freshness and shrivel of Bell pepper fruits. J. Imaging 2020, 6, 122. [Google Scholar] [CrossRef]
- Howard, L.; Talcott, S.; Brenes, C.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef]
- Montet, D.; Ray, R.C.; Zakhia-Rozis, N. Lactic acid fermentation of vegetables and fruits. In Microorganisms and Fermentation of Traditional Foods, 1st ed.; Ray, R.C., Montet, D., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 108–140. [Google Scholar]
- Kim, J.-H.; Block, D.E.; Shoemaker, S.P.; Mills, D.A. Conversion of rice straw to bio-based chemicals: An integrated process using Lactobacillus brevis. Appl. Microbiol. Biotechnol. 2010, 86, 1375–1385. [Google Scholar] [CrossRef] [Green Version]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The Effect of Traditional and Non-Thermal Treatments on the Bioactive Compounds and Sugars Content of Red Bell Pepper. Molecules 2020, 25, 4287. [Google Scholar] [CrossRef]
- USDA5. Peppers, Bell, Green, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2258588/nutrients (accessed on 20 July 2022).
- Verce, M.; De Vuyst, L.; Weckx, S. Comparative genomics of Lactobacillus fermentum suggests a free-living lifestyle of this lactic acid bacterial species. Food Microbiol. 2020, 89, 103448. [Google Scholar] [CrossRef]
- Zhang, Y.; Vadlani, P.V. Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J. Biosci. Bioeng. 2015, 119, 694–699. [Google Scholar] [CrossRef]
- Fu, W.; Mathews, A. Lactic acid production from lactose by Lactobacillus plantarum: Kinetic model and effects of pH, substrate, and oxygen. Biochem. Eng. J. 1999, 3, 163–170. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Barroca, M.J. Effect of drying treatments on texture and color of vegetables (pumpkin and green pepper). Food Bioprod. Process. 2012, 90, 58–63. [Google Scholar] [CrossRef]
- Parniakov, O.; Bals, O.; Lebovka, N.; Vorobiev, E. Effects of pulsed electric fields assisted osmotic dehydration on freezing-thawing and texture of apple tissue. J. Food Eng. 2016, 183, 32–38. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, X.; Yang, J.; Wang, H.; Liu, F.; Xu, X.; Pan, S. Effects of high hydrostatic pressure and thermal treatment on texture properties of pickled kohlrabi. LWT—Food Sci. Technol. 2022, 157, 113078. [Google Scholar] [CrossRef]
- Zi, Y.; Zhixun, S.; Meiqi, L.; Xuetin, Z.; Hongbing, R.; Xiaosong, H.; Junjie, Y. Effect of ripening and variety on the physiochemical quality and flavor of fermented Chinese chili pepper (Paojiao). Food Chem. 2022, 368, 130797. [Google Scholar]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Mousavi Khaneghah, A.; Sant’Ana, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Sano, Y.; Endo, K.; Tomo, T.; Noguchi, T. Modified molecular interactions of the pheophytin and plastoquinone electron acceptors in photosystem II of chlorophyll d-containing Acaryochloris marina as revealed by FTIR spectroscopy. Photosynth. Res. 2015, 125, 105–114. [Google Scholar] [CrossRef]
- Karcz, D.; Boroń, B.; Matwijczuk, A.; Furso, J.; Staroń, J.; Ratuszna, A.; Fiedor, L. Lessons from Chlorophylls: Modifications of Porphyrinoids Towards Optimized Solar Energy Conversion. Molecules 2014, 19, 15938–15954. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.-R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.R.; Perera, M.F.; Arena, M.E. Lactic acid fermentation of peppers. Food Nutr. Sci. 2013, 4, 47–55. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Tracz, K.; Bielińska, P.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Gramza-Michałowska, A. The Impact of the Fermentation Method on the Pigment Content in Pickled Beetroot and Red Bell Pepper Juices and Freeze-Dried Powders. Appl. Sci. 2022, 12, 5766. [Google Scholar] [CrossRef]
- Cierach, M.; Niedźwiedź, J. Effects of three lighting intensities during display on discolouration of beef semitendinosus muscle. Eur. Food Res. Technol. 2014, 239, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochemistry 2019, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, U.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of carotenoids and carotenoid esters in red pepper pods (Capsicum annuum L.) by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2617–2628. [Google Scholar] [CrossRef]
- Huang, S.; Hung, C.; Wu, W.; Chen, B. Determination of chlorophylls and their derivatives in Gynostemma pentaphyllum Makino by liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2008, 48, 105–112. [Google Scholar] [CrossRef]
- Wiktor, A.; Chadzynska, M.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. The Influence of Polyols on the Process Kinetics and Bioactive Substance Content in Osmotic Dehydrated Organic Strawberries. Molecules 2022, 27, 1376. [Google Scholar] [CrossRef]






| Sample Name | Dry Matter (%) | Color Parameters Top | Color Parameters Bottom | Glucose (g/100 g d.m.) | Fructose (g/100 g d.m.) | Total Sugars (g/100 g d.m.) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |||||
| R_Fresh | 6.97 ± 0.14 b | 29.8 ± 0.9 ab | 34.2.4 h | 17.8 ± 1.0 a | 35.9 4.7 d–f | 23.2 ± 1.9 e | 16.9 ± 3.7 a | 22.8 ± 1.3 e | 19.8 ± 0.9 c | 42.5 ± 0.5 g |
| R_LB | 4.57 ± 0.03 ab | 32.2 ± 0.5 ab | 29.3 ± 0.3 g | 32.9 ± 5.7 de | 31.7 ± 1.4 bc | 33.88 ± 3.93 f | 25.10 ± 3.98 bc | 24.6 ± 2.9 e | 12. ± 1.1 b | 36.6 ± 1.8 e |
| R_LF | 4.71 ± 0.36 ab | 34.5 ± 5.0 b | 23.7 ± 4.8 f | 31.1 ± 5.2 cd | 33.9 ± 1.1 a–c | 35.90 ± 1.98 g | 27.38 ± 0.73 cd | 0.00 ± 0.a | 0.2 ± 0.0 a | 0.2 ± 0.0 a |
| R_LP | 4.60 ± 0.03 ab | 29.0 ± 4.0 a | 22.4 ± 3.8 f | 25.7 ±3.7 b | 30.3 ± 3.3 b | 35.08 ± 3.64 g | 31.07 ± 0.33 c–f | 0.0 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 a |
| R_SF | 3.37 ± 0.26 ab | 29.2 ± 2.4 a | 19.6 ± 1.5 e | 21. ± 1.6 a | 25.5 ± 0.5 a | 24.35 ± 0.50 e | 26.85 ± 5.08 cd | 0.0 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 a |
| Y_Fresh | 5.39 ± 0.45 ab | 49.6 ± 3.1 ef | 0.7 ± 0.3 d | 43.9 ± 5.1 g | 42.4 ± 1.7 gh | −0.2 ± 0.7 bc | 25.8 ± 1.3 b–d | 21.8 ± 1.4 e | 17.8 ± 0.5 c | 39.50 ± 0.9 f |
| Y_LB | 5.97 ± 0.39 ab | 51.2 ± 4.4 f | −2.2 ± 0.6 bc | 43.1 ± 6.2 g | 49.0 ± 4.4 j | 2.2 ± 2.5 d | 68.0 ± 10.3 i | 17.8 ± 0.6 d | 11.3 ± 1.3 b | 29.8 ± 0.8 d |
| Y_LF | 4.75 ± 0.22 ab | 44.8 ± 1.0 de | −2.622 ± 0.2 bc | 39.5 ± 0.7 fg | 48.4 ± 2.1 ij | −0.5 ± 0.6 bc | 58.9 ± 1.7 h | 1.9 ± 0.2 ab | 0.5 ± 0.1 a | 2.4 ± 0.3 ab |
| Y_LP | 3.78 ± 1.47 ab | 34.4 ± 6.9 b | −0.712 ± 1.2 cd | 36.5 ± 1.9 ef | 46.2 ± 3.7 i | 1.9 ± 0.2 d | 52.6 ± 11.5 g | 3.9 ± 0.2 b | 0.8 ± 0.1 a | 4.6 ± 0.3 b |
| Y_SF | 4.20 ± 0.40 ab | 42.4 ± 4.78 d | −0.9 ± 0.2 cd | 51.3 ± 5.3 h | 43.3 ± 0.34 | 1.4 ± 1.1 cd | 48.1 ± 1.3 g | 1.4 ± 0.2 ab | 0.6 ± 0.1 a | 1.9 ± 0.2 ab |
| G_Fresh | 4.31 ± 0.35 ab | 33.4 ± 0.9 ab | −12.5 ± 0.5 a | 19.8 ± 1.2 a | 39.3 ± 1.8 fg | −9.9 ± 0.5 a | 17.9 ± 1.2 a | 9.9 ± 0.3 c | 13.9 ± 1.8 b | 23.8 ± 1.4 c |
| G_LB | 3.62 ± 0.18 ab | 35.4 ± 2.0 b | −1.9 ± 0.1 bc | 29.1 ± 0.6 bc | 39.0 ± 1.3 fg | −1.9 ± 0.2 b | 29.1 ± 2.3 c–e | 3.7 ± 0.1 b | 0.3 ± 0.0 a | 4.1 ± 0.1 b |
| G_LF | 3.52 ± 0.25 ab | 33.3 ± 1.9 b | −1.1 ± 0.6 cd | 28.2 ± 0.2 bc | 34.4 ± 2.2 bc | 2.1 ± 0.4 d | 34.4 ± 5.4 ef | 0.0 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 a |
| G_LP | 2.82 ± 0.20 a | 43.7 ± 6.7 d | −3.5 ± 0.8 b | 25.7 ± 0.4 b | 30.8 ± 2.5 b | 1.5 ± 0.2 cd | 29.6 ± 7.3 a–c | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| G_SF | 3.54 ± 0.01 ab | 31.0 ± 2.7 ab | −1.1 ± 0.3 cd | 26.0 ± 0.4 b | 37.4 ± 1.4 ef | 1.0 ± 0.5 b–d | 36.1 ± 1.8 f | 0.0 ± 0.0 a | 0.4 ± 0.0 a | 0.4 ± 0.0 a |
| Sample Name | Hardness [N] | Springiness [%] | Cohesiveness [-] | Chewiness [N] |
|---|---|---|---|---|
| R_Fresh | 34.07 ± 10.97 a–c | 38.94 ± 11.90 ab | 0.33 ± 0.14 a | 3.88 ± 1.87 a–d |
| R_LB | 36.12 ± 9.87 bc | 64.11 ± 14.70 de | 0.19 ± 0.04 a | 4.46 ± 2.37 cd |
| R_LF | 32.89 ± 10.11 ab | 69.14 ± 10.18 de | 0.24 ± 0.11 a | 5.44 ± 1.74 cd |
| R_LP | 35.88 ± 14.03 a–c | 75.58 ± 14.54 e | 0.24 ± 0.13 a | 4.66 ± 2.16 cd |
| R_SF | 27.73 ± 14.34 a–c | 65.99 ± 16.00 b–e | 0.20 ± 0.03 a | 4.30 ± 1.75 a–d |
| Y_Fresh | 22.90 ± 4.74 a | 37.28 ± 19.87 a–c | 0.14 ± 0.05 a | 1.16 ± 0.78 a |
| Y_LB | 29.55 ± 6.48 a | 54.81 ± 13.65 b–d | 0.18 ± 0.04 a | 3.54 ± 1.67 a–c |
| Y_LF | 36.66 ± 6.66 a–c | 62.39 ± 14.08 b–e | 0.25 ± 0.12 a | 5.46 ± 1.89 b–d |
| Y_LP | 33.32 ± 8.88 ab | 62.92 ± 12.97 c–e | 0.26 ± 0.08 a | 6.40 ± 1.98 d |
| Y_SF | 31.04 ± 11.42 a–c | 60.00 ± 17.65 b–e | 0.21 ± 0.10 a | 4.30 ± 2.48 cd |
| G_Fresh | 32.23 ± 3.60 ab | 21.30 ± 11.77 a | 0.18 ± 0.03 a | 1.50 ± 0.87 ab |
| G_LB | 50.47 ± 14.39 c | 57.03 ± 17.04 b–e | 0.18 ± 0.03 a | 4.44 ± 1.68 b–d |
| G_LF | 44.48 ± 13.00 bc | 67.15 ± 15.00 de | 0.31 ± 0.16 a | 6.38 ± 2.69 d |
| G_LP | 44.42 ± 12.28 bc | 67.12 ± 18.01 de | 0.18 ± 0.06 a | 4.78 ± 1.57 cd |
| G_SF | 36.43 ± 15.00 a–c | 66.70 ± 10.99 b–e | 0.22 ± 0.06 a | 5.52 ± 1.70 b–d |
| Sample Name | Chlorophylls and Derivatives | Unbounded Carotenoids | Carotenoid Monoesters | Carotenoid Diesters | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorophyll A | Chlorophyll B | Pheophytin A | Pheophytin B | β-carotene | Capsanthin | Capsorubin | Zeaxanthin | Lutein | β-cryptoxanthin | cap-lau | cap-myr | cap-pal | zea-lau | zea-myr | zea-pal | cap-lau-lau | cap-lau-myr | cap-myr-myr | cap-lau-pal | cap-myr-pal | cap-pal-pal | zea-myr-myr | zea-lau-pal | zea-myr-pal | zea-pal-pal | |
| R_Fresh | 0 | 0 | 0 | 0 | 7.92 ± 0.22 f | 9.49 ± 0.43 a | 50.44 ± 1.97 c | 45.33 ± 1.07 a | 19.64 ± 0.96 a | 9.40 ± 0.31 a | 0.58 ± 0.03 a | 2.67 ± 0.05 a | 13.98 ± 0.27 b | 0.34 ± 0.04 a | 0.66 ± 0.04 a | 2.09 ± 0.07 a | 0.10 ± 0.02 a | 0.42 ± 0.03 a | 0.50 ± 0.02 a | 0.24 ± 0.00 a | 0.98 ± 0.05 a | 5.74 ± 0.02 b | 0.30 ± 0.02 a | 0.23 ± 0.00 a | 0.48 ± 0.05 a | 1.45 ± 0.02 a |
| R_LB | 0 | 0 | 0 | 0 | 12.39 ± 0.14 h | 12.72 ± 0.05 c | 77.42 ± 0.88 fg | 67.26 ± 0.99 b | 28.26 ± 0.14 b | 13.48 ± 0.45 c | 0.73 ± 0.02 b | 3.89 ± 0.06 b | 20.89 ± 0.14 d | 0.49 ± 0.05 b | 0.91 ± 0.14 b | 3.07 ± 0.05 c | 0.16 ± 0.02 b | 0.54 ± 0.08 b | 0.63 ± 0.03 bc | 0.31 ± 0.06 a–c | 1.45 ± 0.08 c | 8.34 ± 0.26 d | 0.42 ± 0.03 b | 0.36 ± 0.02 c | 0.68 ± 0.09 bc | 2.22 ± 0.08 bc |
| R_LF | 0 | 0 | 0 | 0 | 11.09 ± 0.47 g | 12.07 ± 0.08 b | 71.20 ± 1.10 ef | 67.01 ± 1.32 b | 27.43 ± 0.15 b | 13.34 ± 0.20 c | 0.76 ± 0.06 b | 3.63 ± 0.12 b | 20.33 ± 0.44 d | 0.46 ± 0.08 b | 0.93 ± 0.06 b | 2.97 ± 0.03 b | 0.15 ± 0.00 b | 0.49 ± 0.03 ab | 0.61 ± 0.02 b | 0.35 ± 0.02 c | 1.40 ± 0.06 c | 7.45 ± 0.21 c | 0.41 ± 0.02 b | 0.32 ± 0.03 bc | 0.63 ± 0.11 ab | 2.12 ± 0.06 cd |
| R_LP | 0 | 0 | 0 | 0 | 12.35 ± 0.58 h | 12.79 ± 0.3 c | 75.78 ± 0.43 fg | 67.85 ± 0.74 b | 28.68 ± 0.63 b | 13.96 ± 0.28 c | 0.78 ± 0.03 b | 3.90 ± 0.08 b | 20.58 ± 0.11 d | 0.48 ± 0.00 b | 0.95 ± 0.02 b | 3.16 ± 0.05 c | 0.14 ± 0.02 b | 0.58 ± 0.02 b | 0.70 ± 0.03 c | 0.32 ± 0.02 bc | 1.46 ± 0.00 c | 8.42 ± 0.02 d | 0.39 ± 0.03 b | 0.32 ± 0.02 bc | 0.71 ± 0.02 c | 2.28 ± 0.03 d |
| R_SF | 0 | 0 | 0 | 0 | 12.41 ± 0.06 h | 12.62 ± 0.31 bc | 85.68 ± 3.19 h | 76.80 ± 3.50 c | 32.32 ± 0.42 c | 17.36 ± 0.06 h | 0.53 ± 0.04 a | 2.83 ± 0.15 a | 17.78 ± 0.49 c | 0.46 ± 0.06 b | 0.90 ± 0.02 b | 2.85 ± 0.17 b | 0.15 ± 0.00 b | 0.53 ± 0.04 b | 0.68 ± 0.04 c | 0.25 ± 0.02 ab | 1.25 ± 0.04 b | 8.33 ± 0.29 d | 0.36 ± 0.04 ab | 0.27 ± 0.04 ab | 0.53 ± 0.04 ab | 1.97 ± 0.10 b |
| Y_Fresh | 0 | 0 | 0.37 ± 0.08 a | 0 | 2.19 ± 0.24 c–e | 0 | 58.57 ± 2.18 d | 0 | 0 | 15.01 ± 0.05 ef | 0 | 0 | 0.20 ± 0.03 a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.35 ± 0.03 a | 0 | 0 | 0 | 0 |
| Y_LB | 0 | 0 | 0.31 0.04 a | 0 | 1.92 ± 0.06 a–e | 0 | 49.07 ± 0.82 c | 0 | 0 | 13.47 ± 0.14 c | 0 | 0 | 0.14 ± 0.01 a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.25 ± 0.02 a | 0 | 0 | 0 | 0 |
| Y_LF | 0 | 0 | 0.40 0.02 a | 0 | 2.41 ± 0.09 de | 0 | 65.30 ± 4.30 de | 0 | 0 | 17.09 ± 0.06 h | 0 | 0 | 0.20 ± 0.00 a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.37 ± 0.00 a | 0 | 0 | 0 | 0 |
| Y_LF | 0 | 0 | 0.48 0.04 a | 0 | 3.01 ± 0.19 e | 0 | 79.44 ± 0.65 g | 0 | 0 | 21.05 ± 0.22 j | 0 | 0 | 0.25 ± 0.02 a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.41 ± 0.02 a | 0 | 0 | 0 | 0 |
| Y_SF | 0 | 0 | 0.43 0.07 a | 0 | 2.27 ± 0.05 c–e | 0 | 64.20 ± 0.57 de | 0 | 0 | 18.02 ± 0.10 i | 0 | 0 | 0.18 ± 0.02 a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.20 ± 0.02 a | 0 | 0 | 0 | 0 |
| G_Fresh | 139.20 ± 14.79 c | 16.66 ± 1.52 b | 9.97 ± 0.51 a | 4.43 ± 0.70 a | 1.24 ± 0.06 ab | 0 | 18.84 ± 0.55 a | 0 | 0 | 11.98 ± 0.28 b | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G_LB | 0.57 ± 0.10 a | 0 | 182.85 ± 11.44 bc | 22.61 ± 0.16 b | 1.41 ± 0.04 ab | 0 | 25.31 ± 0.18 ab | 0 | 0 | 15.43 ± 0.12 fg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G_LF | 0 | 0 | 183.47 ± 6.27 bc | 22.07 ± 0.50 b | 1.46 ± 0.10 a–c | 0 | 24.65 ± 2.13 ab | 0 | 0 | 16.16 ± 0.06 g | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G_LP | 17.31 ± 1.23 b | 1.54 ± 0.18 a | 193.90 ± 2.26 c | 26.78 ± 0.58 c | 2.01 ± 0.1 b–d | 0 | 31.10 ± 1.05 b | 0 | 0 | 20.54 ± 0.08 j | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G_SF | 0 | 0 | 176.20 ± 10.84 b | 21.75 ± 1.18 b | 1.03 ± 0.06 a | 0 | 21.13 0.18 a | 0 | 0 | 14.30 ± 0.10 de | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiszewska-Turak, E.; Witrowa-Rajchert, D.; Rybak, K.; Rolof, J.; Pobiega, K.; Woźniak, Ł.; Gramza-Michałowska, A. The Influence of Lactic Acid Fermentation on Selected Properties of Pickled Red, Yellow, and Green Bell Peppers. Molecules 2022, 27, 8637. https://doi.org/10.3390/molecules27238637
Janiszewska-Turak E, Witrowa-Rajchert D, Rybak K, Rolof J, Pobiega K, Woźniak Ł, Gramza-Michałowska A. The Influence of Lactic Acid Fermentation on Selected Properties of Pickled Red, Yellow, and Green Bell Peppers. Molecules. 2022; 27(23):8637. https://doi.org/10.3390/molecules27238637
Chicago/Turabian StyleJaniszewska-Turak, Emilia, Dorota Witrowa-Rajchert, Katarzyna Rybak, Joanna Rolof, Katarzyna Pobiega, Łukasz Woźniak, and Anna Gramza-Michałowska. 2022. "The Influence of Lactic Acid Fermentation on Selected Properties of Pickled Red, Yellow, and Green Bell Peppers" Molecules 27, no. 23: 8637. https://doi.org/10.3390/molecules27238637