Photocatalytic Aerobic Oxidation of Biomass-Derived 5-HMF to DFF over MIL-53(Fe)/g-C3N4 Composite
Abstract
:1. Introduction
2. Results and Discussions
2.1. Catalyst Characterizations
2.1.1. Structure Characterization
2.1.2. Morphology Characterization
2.1.3. Optical Property Characterization
2.1.4. XPS Characterization
2.1.5. PL, EIS and PC Characterization
2.2. Photocatalytic Performance
2.2.1. The Effect of Incident Light
2.2.2. The Effect of MIL-53(Fe) Content
2.2.3. The Effect of O2 Flow Rate
2.2.4. The Effect of Solvent
2.2.5. Recycle Test
2.3. Mechanism
2.3.1. The Effect of Active Species
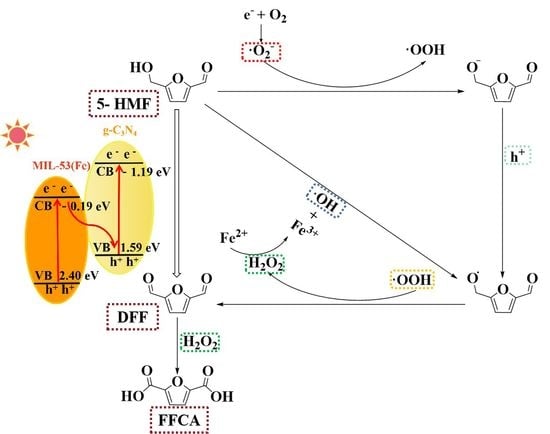
2.3.2. Possible Photocatalytic Mechanism

3. Experimental
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Photocatalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent Catalytic Routes for the Preparation and the Upgrading of Biomass Derived Furfural and 5-Hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, R.J.; Van De Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Rosatella, A.A.; Simeonov, S.P.; Fradea, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a Building Block Platform: Biological Properties, Synthesis and Synthetic Applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Takagaki, A.; Takahashi, M.; Nishimura, S.; Ebitani, K. One-Pot Synthesis of 2,5-Diformylfuran from Carbohydrate Derivatives by Sulfonated Resin and Hydrotalcite-Supported Ruthenium Catalysts. ACS Catal. 2011, 1, 1562–1565. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, J.; Guo, Y.; Chen, L. Ruthenium Complex Immobilized on Poly(4-Vinylpyridine)-Functionalized Carbon-Nanotube for Selective Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran. RSC Adv. 2015, 5, 5933–5940. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Deng, J.; Pan, T.; Guo, Q.; Fu, Y. A One-Pot Approach for Conversion of Fructose to 2,5-Diformylfuran by Combination of Fe3O4-SBA-SO3H and K-OMS-2. Green Chem. 2012, 14, 2986–2989. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Stepurko, E.N.; Ralys, R.V.; Zaitsau, D.H.; Stark, A. Biomass-Derived Platform Chemicals: Thermodynamic Studies on the Conversion of 5-Hydroxymethylfurfural into Bulk Intermediates. Ind. Eng. Chem. Res. 2009, 48, 10087–10093. [Google Scholar] [CrossRef]
- Navarro, O.C.; Canos, A.C.; Chornet, S.I. Chemicals from Biomass: Aerobic Oxidation of 5-Hydroxymethyl-2-Furaldehyde into Diformylfurane Catalyzed by Immobilized Vanadyl-Pyridine Complexes on Polymeric and Organofunctionalized Mesoporous Supports. Top. Catal. 2009, 52, 304–314. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Karuppasamy, K.; Lee, S.J.; Shwetharani, R.; Kim, H.-S.; Khadheer Pasha, S.K.; Ashokkumar, M.; Choi, M.Y. Fundamentals and Comprehensive Insights on Pulsed Laser Synthesis of Advanced Materials for Diverse Photo- and Electrocatalytic Applications. Light Sci. Appl. 2022, 11, 250. [Google Scholar] [CrossRef]
- Lee, S.J.; Theerthagiri, J.; Nithyadharseni, P.; Arunachalam, P.; Balaji, D.; Kumar, A.M.; Madhavan, J.; Mittal, V.; Choi, M.Y. Heteroatom-Doped Graphene-Based Materials for Sustainable Energy Applications: A Review. Renew. Sust. Energ. Rev. 2021, 143, 110849. [Google Scholar] [CrossRef]
- Yurdakal, S.; Tek, B.S.; Alagoz, O.; Augugliaro, V.; Loddo, V.; Palmisano, G.; Palmisano, L. Photocatalytic Selective Oxidation of 5-(Hydroxymethyl)-2-Furaldehyde to 2,5-Furandicarbaldehyde in Water by Using Anatase, Rutile, and Brookite TiO2 Nanoparticles. ACS Sustain. Chem. Eng. 2013, 1, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Goepel, M.; Kubas, A.; Lomot, D.; Lisowski, W.; Lisovytskiy, D.; Nowicka, A.; Colmenares, J.C.; Glaser, R. Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran by Visible Light-Driven Photocatalysis over in-Situ Substrate-Sensitized Titania. ChemSusChem 2021, 14, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Allegri, A.; Maslova, V.; Blosi, M.; Costa, A.L.; Ortelli, S.; Basile, F.; Albonetti, S. Photocatalytic Oxidation of HMF under Solar Irradiation: Coupling of Microemulsion and Lyophilization to Obtain Innovative TiO2-Based Materials. Molecules 2020, 25, 5225. [Google Scholar] [CrossRef] [PubMed]
- Krivtsov, I.; Ilkaeva, M.; Salas-Colera, E.; Amghouz, Z.; Garcia, J.R.; Diaz, E.; Ordonez, S.; Villar-Rodil, S. Consequences of Nitrogen Doping and Oxygen Enrichment on Titanium Local Order and Photocatalytic Performance of TiO2 Anatase. J. Phys. Chem. C 2017, 121, 6770–6780. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Song, J.; Zhang, Z.; Jiang, Z.; Zhang, P.; Han, B. Highly Selective Photocatalytic Oxidation of Biomass-Derived Chemicals to Carboxyl Compounds over Au/TiO2. Green Chem. 2017, 19, 1075. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wu, Q.; Guo, C.; Wu, Y.; Wu, T. Photocatalytic Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran over Nb2O5 under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 3517–3523. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Nair, V.; Khan, A.; Deliyanni, E.A.; Colmenares, J.C.; Triantafyllidis, K.S. Additive-Free Photo-Assisted Selective Partial Oxidation at Ambient Conditions of 5-Hydroxymethylfurfural by Manganese (IV) Oxide Nanorods. Appl. Catal. B Environ. 2019, 256, 117803. [Google Scholar] [CrossRef]
- Gonzalez-Casamachina, D.A.; De La Rosaa, J.R.; Lucio-Ortiza, C.J.; Sandoval-Rangel, L.; Garcia, C.D. Partial Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid Using O2 and a Photocatalyst of a Composite of ZnO/PPy under Visible-Light: Electrochemical Characterization and Kinetic Analysis. Chem. Eng. J. 2020, 393, 124699. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R. Heterogeneous Photocatalytic Nanomaterials: Prospects and Challenges in Selective Transformations of Biomass-Derived Compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef]
- Gong, Y.; Li, M.; Li, H.; Wang, Y. Graphitic Carbon Nitride Polymers: Promising Catalysts or Catalyst Supports for Heterogeneous Oxidation and Hydrogenation. Green Chem. 2015, 17, 715–736. [Google Scholar] [CrossRef]
- Krivtsov, I.; Garcia-Lopez, E.I.; Marci, G.; Palmisano, L.; Amghouz, Z.; Garcia, J.R.; Ordonez, S.; Diaz, E. Selective Photocatalytic Oxidation of 5-Hydroxymethyl-2-Furfural to 2,5-Furandicarboxyaldehyde in Aqueous Suspension of g-C3N4. Appl. Catal. B Environ. 2017, 204, 430–439. [Google Scholar] [CrossRef]
- Wu, Q.; He, Y.; Zhang, H.; Feng, Z.; Wu, Y.; Wu, T. Photocatalytic Selective Oxidation of Biomass-Derived 5-Hydroxymethylfurfural to 2,5-Diformylfuran on Metal-Free g-C3N4 under Visible Light Irradiation. Mol. Catal. 2017, 436, 10–18. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Cheng, L.; Ismael, M.; Feng, Z.; Wu, Y. Novel Application of g-C3N4/NaNbO3 Composite for Photocatalytic Selective Oxidation of Biomass-Derived HMF to FFCA under Visible Light Irradiation. Adv. Powder Technol. 2020, 31, 1148–1159. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, P.; Zhang, Z.; Yang, C.; Zhang, B.; Deng, K.; Bottle, S.; Zhu, H. Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid Using O2 and a Photocatalyst of Co-Thioporphyrazine Bonded to g-C3N4. J. Am. Chem. Soc. 2017, 139, 14775–14782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, Z.; Zhu, Y.; Wu, Y.; Wu, T. Photocatalytic Selective Oxidation of Biomass-Derived 5-Hydroxymethylfurfural to 2,5-Diformylfuran on WO3/g-C3N4 Composite under Irradiation of Visible Light. J. Photochem. Photobiol. A Chem. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, D.; Zhang, Y.; Wu, Y. Photocatalytic Selective Oxidation of HMF to DFF over Bi2WO6/mpg-C3N4 Composite under Visible Light. Appl. Organomet. Chem. 2021, 35, e6404. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydin, A.O.; Hupp, J.T. Metal-Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit. J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Vilela, S.M.F.; Tome, J.P.C.; Paz, F.A.A. Multifunctional Metal-Organic Frameworks: From Academia to Industrial Applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Herbst, A.; Janiak, C. MOF Catalysts in Biomass Upgrading towards Value-Added Fine Chemicals. Crystengcomm 2016, 19, 4092–4117. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.; Jiang, H. Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, L.; Cohen, S.M. Photocatalytic Metal–Organic Frameworks for Organic Transformations. Crystengcomm 2017, 19, 4126. [Google Scholar] [CrossRef]
- Xiao, J.; Jiang, H. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef]
- Bai, C.; Bi, J.; Wu, J.; Meng, H.; Xu, Y.; Han, Y.; Zhang, X. Fabrication of Noble-Metal-Free g-C3N4-MIL-53(Fe) Composite for Enhanced Photocatalytic H2-Generation Performance. Appl. Organomet. Chem. 2018, 32, e4597. [Google Scholar] [CrossRef]
- Huang, W.; Liu, N.; Zhang, X.; Wu, M.; Tang, L. Metal Organic Framework g-C3N4/MIL-53(Fe) Heterojunctions with Enhanced Photocatalytic Activity for Cr(VI) Reduction under Visible Light. Appl. Surf. Sci. 2017, 425, 107–116. [Google Scholar] [CrossRef]
- Panda, R.; Rahut, S.; Basu, J.K. Preparation of a Fe2O3/MIL-53(Fe) Composite by Partial Thermal Decomposition of MIL-53(Fe) Nanorods and Their Photocatalytic Activity. RSC Adv. 2016, 6, 80981–80985. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, D.; Ren, H.; An, K.; Chen, Y.; Zhou, Z.; Wang, W.; Jiang, Z. Nitrogenase-Inspired Mixed-Valence MIL-53(FeII/FeIII) for Photocatalytic Nitrogen Fixation. Chem. Eng. J. 2020, 400, 125929. [Google Scholar] [CrossRef]
- Yan, S.; Li, Z.; Zou, Z. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Dung, N.T.; Van Hiep, N.; Nguyen, M.B.; Thao, V.D.; Huy, N.N. Photocatalytic Removal of Rhodamine B in Water Using g-C3N4/MIL-53(Fe) Material under LED Visible Light with Persulfate Activation. Korean J. Chem. Eng. 2021, 38, 2034–2046. [Google Scholar] [CrossRef]
- Liu, M.; Xia, P.; Zhang, L.; Cheng, B.; Yu, J. Enhanced Photocatalytic H2 Production Activity of g-C3N4 Nanosheets via Optimal Photodeposition of Pt as Cocatalyst. ACS Sustain. Chem. Eng. 2018, 6, 10472–10480. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, W. Photocatalytic Hydrogen Evolution over a Nickel Complex Anchoring to Thiophene Embedded g-C3N4. J. Colloid Interf. Sci. 2021, 596, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Shen, L.; Jiang, L. Amino-Modified Fe-Terephthalate Metal-Organic Framework as an Efficient Catalyst for the Selective Oxidation of H2S. Inorg. Chem. 2018, 57, 10081–10089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Y.; Hao, D.; Guo, J.; Ni, B.J.; Jiang, B.; Xu, L.; Wang, Q. Fabrication of CN75/NH2-MIL-53(Fe) p-n Heterojunction with Wide Spectral Response for Efficiently Photocatalytic Cr(VI) Reduction. J. Alloys Compd. 2021, 891, 161994. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z. Iron-Based Metal–Organic Frameworks (MOFs) for Visible-Light-Induced Photocatalysis. Res. Chem. Intermed. 2017, 43, 5169–5186. [Google Scholar] [CrossRef]
- López, J.; Chávez, A.M.; Rey, A.; Álvarez, P.M. Insights into the Stability and Activity of MIL-53(Fe) in Solar Photocatalytic Oxidation Processes in Water. Catalysts 2021, 11, 448. [Google Scholar] [CrossRef]











| Entry | Conditions | Conv. of 5-HMF (%) | Sel. to DFF (%) | Yield to DFF (%) |
|---|---|---|---|---|
| 1 | No catalyst | 0 | 0 | 0 |
| 2 | Dark | 0 | 0 | 0 |
| 3 | λ > 360 nm | 74.5 | 45.9 | 34.1 |
| 5 | λ > 400 nm | 29.5 | 59.6 | 17.6 |
| 6 | λ > 420 nm | 14.2 | 66.3 | 9.4 |
| Entry | Gas | Flow Rate (mL/min) | Conv. of 5-HMF (%) | Sel. to DFF (%) | Yield to DFF (%) |
|---|---|---|---|---|---|
| 1 | O2 | 2.5 | 45.9 | 19.8 | 9.1 |
| 2 | O2 | 5 | 51.7 | 34.1 | 17.6 |
| 3 | O2 | 10 | 74.5 | 45.9 | 34.1 |
| 4 | O2 | 15 | 76.5 | 48.5 | 37.1 |
| 5 | N2 | 10 | 4.3 | 31.5 | 1.4 |
| 6 | Air | 10 | 43.6 | 20.2 | 8.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Wang, H.; Wu, Y. Photocatalytic Aerobic Oxidation of Biomass-Derived 5-HMF to DFF over MIL-53(Fe)/g-C3N4 Composite. Molecules 2022, 27, 8537. https://doi.org/10.3390/molecules27238537
Huang D, Wang H, Wu Y. Photocatalytic Aerobic Oxidation of Biomass-Derived 5-HMF to DFF over MIL-53(Fe)/g-C3N4 Composite. Molecules. 2022; 27(23):8537. https://doi.org/10.3390/molecules27238537
Chicago/Turabian StyleHuang, Danyao, Hao Wang, and Ying Wu. 2022. "Photocatalytic Aerobic Oxidation of Biomass-Derived 5-HMF to DFF over MIL-53(Fe)/g-C3N4 Composite" Molecules 27, no. 23: 8537. https://doi.org/10.3390/molecules27238537