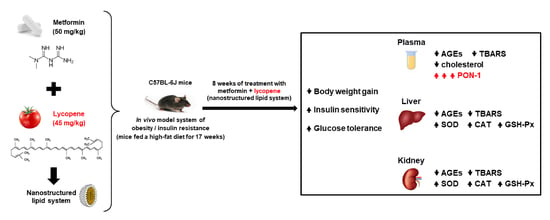
Combined Effects of Lycopene and Metformin on Decreasing Oxidative Stress by Triggering Endogenous Antioxidant Defenses in Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Results
2.1. Lycopene Combined with Metformin Reduced Body Weight Gain and Fat Deposition in Obese Mice
2.2. Lycopene Combined with Metformin Improved Insulin Sensitivity and Glucose Tolerance in Obese Mice
2.3. Lycopene Combined with Metformin Reduced Glycoxidative Stress in Plasma and Significantly Increased the Activity of Paraoxonase-1 (PON-1) in Obese Mice
2.4. Lycopene Combined with Metformin Triggered Endogenous Antioxidant Defenses in Liver and Kidneys of Obese Mice
3. Discussion
4. Materials and Methods
4.1. Nanostructured Lipid System Preparation
4.2. Animal Experimental Design
4.3. Plasma Biochemical Analysis
4.4. Biomarkers of Glycoxidative Stress
4.5. Analysis of the Antioxidant Defenses
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.G.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 11 September 2022).
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free. Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.H.; Ramasamy, R.; Schmidt, A.M. Advanced Glycation End Products: Building on the Concept of the “Common Soil” in Metabolic Disease. Endocrinology 2019, 161, bqz006. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’Nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [Green Version]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association 7. Approaches to Glycemic Treatment. Diabetes Care 2014, 38, S41–S48. [Google Scholar] [CrossRef] [Green Version]
- Piskovatska, V.; Storey, K.B.; Vaiserman, A.M.; Lushchak, O. The Use of Metformin to Increase the Human Healthspan. In Reviews on New Drug Targets in Age-Related Disorders; eBook; Guest, P.C., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 319–332. [Google Scholar] [CrossRef]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic Durability of Rosiglitazone, Metformin, or Glyburide Monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443. [Google Scholar] [CrossRef]
- DeFronzo, R.A. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [Green Version]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef]
- Albrahim, T.; Alonazi, M.A. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed. Pharmacother. 2021, 141, 111831. [Google Scholar] [CrossRef]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.; Al-Shali, K.Z.; Ahmad, S. Lycopene powers the inhibition of glycation-induced diabetic nephropathy: A novel approach to halt the AGE-RAGE axis menace. BioFactors 2015, 41, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhu, R.; Zhang, H.; Chen, B.; Liu, Y.; Dai, X.; Ye, Z.; Zhao, D.; Mo, F.; Gao, S.; et al. Lycopene Improves Bone Quality and Regulates AGE/RAGE/NF-кB Signaling Pathway in High-Fat Diet-Induced Obese Mice. Oxidative Med. Cell. Longev. 2022, 2022, 3697067. [Google Scholar] [CrossRef]
- Cahn, A.; Cefalu, W.T. Clinical Considerations for Use of Initial Combination Therapy in Type 2 Diabetes. Diabetes Care 2016, 39, S137–S145. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, A.; Bagherniya, M.; Mirenayat, M.S.; Atkin, S.L.; Sahebkar, A. Medicinal Plants and Phytochemicals Regulating Insulin Resistance and Glucose Homeostasis in Type 2 Diabetic Patients: A Clinical Review. In Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health; Barreto, G.E., Sahebkar, A., Eds.; Springer: Cham, Switzerland, 2021; Volume 1308, pp. 161–183. [Google Scholar] [CrossRef]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; López Machado, A.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M.; et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Review of Classical and New Compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, I.D.; Lima, T.F.O.; Inácio, M.D.; Costa, M.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Lycopene Improves the Metformin Effects on Glycemic Control and Decreases Biomarkers of Glycoxidative Stress in Diabetic Rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3117–3135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.-D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Ozmen, O.; Topsakal, S.; Haligur, M.; Aydogan, A.; Dincoglu, D. Effects of Caffeine and Lycopene in Experimentally Induced Diabetes Mellitus. Pancreas 2016, 45, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Schommers, P.; Thurau, A.; Bultmann-Mellin, I.; Guschlbauer, M.; Klatt, A.R.; Rozman, J.; Klingenspor, M.; de Angelis, M.H.; Alber, J.; Gründemann, D.; et al. Metformin causes a futile intestinal–hepatic cycle which increases energy expenditure and slows down development of a type 2 diabetes-like state. Mol. Metab. 2017, 6, 737–747. [Google Scholar] [CrossRef]
- Albrahim, T.; Robert, A.A. Lycopene Effects on Metabolic Syndrome and Kidney Injury in Rats Fed a High-Fat Diet: An Experimental Study. ACS Omega 2022, 7, 30930–30938. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wei, J.; Liu, H.; Liu, C.; Wang, L.; Chen, B.; Li, L.; Jia, Q.; Tian, Y.; Li, R.; et al. Lycopene attenuates body weight gain through induction of browning via regulation of peroxisome proliferator-activated receptor γ in high-fat diet-induced obese mice. J. Nutr. Biochem. 2020, 78, 108335. [Google Scholar] [CrossRef] [PubMed]
- Szaleczky, E.; Prechl, J.; Fehér, J.; Somogyi, A. Alterations in enzymatic antioxidant defence in diabetes mellitus—A rational approach. Postgrad. Med. J. 1999, 75, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Daffu, G.; Del Pozo, C.H.; O’Shea, K.M.; Ananthakrishnan, R.; Ramasamy, R.; Schmidt, A.M. Radical Roles for RAGE in the Pathogenesis of Oxidative Stress in Cardiovascular Diseases and Beyond. Int. J. Mol. Sci. 2013, 14, 19891–19910. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Feng, J.; Peng, Q.; Liu, X.; Fan, Z. Advanced Glycation End Products: Potential Mechanism and Therapeutic Target in Cardiovascular Complications under Diabetes. Oxidative Med. Cell. Longev. 2019, 2019, 9570616. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhang, J.; Cao, Z.; Ji, Q.; Han, Y.; Song, M.; Shao, B.; Li, Y. Lycopene attenuates AFB1-induced renal injury with the activation of the Nrf2 antioxidant signaling pathway in mice. Food Funct. 2018, 9, 6427–6434. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Ma, J.; Xv, Q.; Gao, H.; Yin, H.; Yan, G.; Jiang, X.; Yu, W. Lycopene attenuates the inflammation and apoptosis in aristolochic acid nephropathy by targeting the Nrf2 antioxidant system. Redox Biol. 2022, 57, 102494. [Google Scholar] [CrossRef] [PubMed]
- Otocka-Kmiecik, A. Effect of Carotenoids on Paraoxonase-1 Activity and Gene Expression. Nutrients 2022, 14, 2842. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Melnichenko, A.A.; Orekhov, A.N.; Bobryshev, Y.V. Paraoxonase and atherosclerosis-related cardiovascular diseases. Biochimie 2017, 132, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.G.; Barros, A.L.A.N.; Cabral, W.F.; Moreira, D.C.; da Silva, I.G.M.; Silva-Carvalho, A.; de Almeida, M.P.; Albuquerque, L.F.F.; dos Santos, R.C.; Brito, A.K.S.; et al. Promising self-emulsifying drug delivery system loaded with lycopene from red guava (Psidium guajava L.): In vivo toxicity, biodistribution and cytotoxicity on DU-145 prostate cancer cells. Cancer Nanotechnol. 2021, 12, 30. [Google Scholar] [CrossRef]
- Buse, J.B.; DeFronzo, R.A.; Rosenstock, J.; Kim, T.; Burns, C.; Skare, S.; Baron, A.; Fineman, M. The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation. Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes Care 2015, 39, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, G.C.; de Camargo, B.A.F.; de Araújo, J.T.C.; Chorilli, M. Lycopene: From tomato to its nutraceutical use and its association with nanotechnology. Trends Food Sci. Technol. 2021, 118, 447–458. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Rostamabadi, H.; Babazadeh, A.; Tarhan, Ö.; Rashidinejad, A.; Boostani, S.; Khoshnoudi-Nia, S.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Lycopene nanodelivery systems; recent advances. Trends Food Sci. Technol. 2021, 119, 378–399. [Google Scholar] [CrossRef]
- Leh, H.E.; Sopian, M.M.; Abu Bakar, M.H.; Lee, L.K. The role of lycopene for the amelioration of glycaemic status and peripheral antioxidant capacity among the Type II diabetes mellitus patients: A case–control study. Ann. Med. 2021, 53, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, E.S.; Silva, P.; Chorilli, M.; Batista, A.A.; de Oliveira Lopes, E.; Da Silva, M.M.; Leite, C.Q.F.; Pavan, F.R. Nanostructured Lipid Systems as a Strategy to Improve the in Vitro Cytotoxicity of Ruthenium(II) Compounds. Molecules 2014, 19, 5999–6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talpo, T.C.; Motta, B.P.; de Oliveira, J.O.; Figueiredo, I.D.; Pinheiro, C.G.; dos Santos, C.H.C.; de Carvalho, M.G.; Brunetti, I.L.; Baviera, A.M. Siolmatra brasiliensis stem extract ameliorates antioxidant defenses and mitigates glycoxidative stress in mice with high-fat diet-induced obesity. Obes. Res. Clin. Pract. 2022, 16, 130–137. [Google Scholar] [CrossRef]
- Assis, R.P.; Arcaro, C.A.; Gutierres, V.O.; Oliveira, J.O.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 332. [Google Scholar] [CrossRef] [Green Version]
- Zilin, S.; Naifeng, L.; Bicheng, L.; Jiping, W. The determination of AGE-peptides by flow injection assay, a practical marker of diabetic nephropathy. Clin. Chim. Acta 2001, 313, 69–75. [Google Scholar] [CrossRef]
- Pokupec, R.; Kalauz, M.; Turk, N.; Turk, Z. Advanced glycation endproducts in human diabetic and non-diabetic cataractous lenses. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 378–384. [Google Scholar] [CrossRef]
- Kohn, H.I.; Liversedge, M. On a new aerobic metabolite whose production by brain is inhibited by apomorphine, emetine, ergotamine, epinephrine, and menadione. J. Pharmacol. Exp. Ther. 1944, 82, 292–300. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Beers, R.F., Jr.; Sizer, I.W. A spectrophotometric method of measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.W.; Sandiford, S.D. Plasma glutathione peroxidase in healthy young adults: Influence of gender and physical activity. Clin. Biochem. 2003, 36, 345–351. [Google Scholar] [CrossRef]
- Costa, L.G.; McDonald, B.E.; Murphy, S.D.; Omenn, G.S.; Richter, R.J.; Motulsky, A.G.; Furlong, C.E. Serum paraoxonase and its influence on paraoxon and chlorpyrifos-oxon toxicity in rats. Toxicol. Appl. Pharmacol. 1990, 103, 66–76. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]





| Groups | C | H | H-V | H-M | H-L | H-ML |
|---|---|---|---|---|---|---|
| Body weight (g) at week 0 (beginning of experiment) | 21.77 ± 0.421 | 22.04 ± 0.412 | 21.74 ± 0.275 | 22.25 ± 0.563 | 22.39 ± 0.559 | 21.65 ± 0.413 |
| Body weight (g) at week 9 (beginning of treatments) | 26.90 ± 0.412 (#) | 31.30 ± 0.546 (#,a) | 30.02 ± 0.712 (#,a) | 30.01 ± 0.719 (#,a) | 30.11 ± 0.697 (#,a) | 30.27 ± 0.413 (#,a) |
| Body weight gain (g) (week 0 → week 9) | 5.12 ± 0.525 | 9.26 ± 0.398 | 8.71 ± 0.638 | 7.76 ± 0.635 | 7.71 ± 0.440 | 8.61 ± 0.523 |
| Body weight (g) at week 17 (end of treatments) | 26.74 ± 0.477 (#) | 33.88 ± 0.744 (#,a) | 32.36 ± 0.633 (#,a) | 28.87 ± 0.749 (#,b,c) | 27.55 ± 0.351 (#,b,c) | 28.62 ± 0.495 (#,b,c) |
| Body weight gain (g) (week 9 → week 17) | −0.16 ± 0.361 | 2.73 ± 0.387 | 2.49 ± 0.636 | −1.15 ± 0.482 | −2.65 ± 0.729 | −1.63 ± 0.480 |
| Groups | C | H | H-V | H-M | H-L | H-ML |
|---|---|---|---|---|---|---|
| Epididymal WAT (mg/mm tibia) | 26.74 ± 1.474 | 73.04 ± 5.432 (a) | 53.74 ± 3.980 (a,b) | 37.20 ± 3.262 (b,c) | 36.44 ± 2.89 (b,c) | 37.97 ± 3.243 (b,c) |
| Retroperitoneal WAT (mg/mm tibia) | 11.30 ± 0.798 | 30.62 ± 1.797 (a) | 21.52 ± 1.775 (a,b) | 14.02 ± 1.257 (b,c) | 12.78 ± 0.853 (b,c) | 15.13 ± 1.213 (b,c) |
| Interscapular BAT (mg/mm tibia) | 3.72 ± 0.144 | 3.97 ± 0.311 | 3.56 ± 0.240 | 3.17 ± 0.204 | 3.31 ± 0.251 | 3.34 ± 0.218 |
| Liver (mg/mm tibia) | 57.95 ± 0.872 | 67.16 ± 1.573 (a) | 62.87 ± 2.126 (a) | 57.37 ± 1.805 (b,c) | 57.08 ± 1380 (b,c) | 57.64 ± 1.492 (b,c) |
| Kidney (mg/mm tibia) | 8.23 ± 0.537 | 10.98 ± 0.656 (a) | 10.10 ± 0.766 (a) | 8.78 ± 0.579 (b,c) | 8.88 ± 0.748 (b,c) | 8.79 ± 0.395 (b,c) |
| Heart (mg/mm tibia) | 7.08 ± 0.206 | 7.22 ± 0.108 | 7.11 ± 0.172 | 7.13 ± 0.160 | 7.04 ± 0.140 | 7.20 ± 0.180 |
| Gastrocnemius muscle (mg/mm tibia) | 8.08 ± 0.079 | 8.31 ± 0.174 | 8.11 ± 0.140 | 8.12 ± 0.103 | 8.16 ± 0.103 | 8.31 ± 0.152 |
| Groups | C | H | H-V | H-M | H-L | H-ML |
|---|---|---|---|---|---|---|
| Triglycerides (mg/dL) | 52.1 ± 20.6 | 41.2 ± 2.08 (a) | 40.0 ± 2.37 (a) | 38.9 ± 3.65 (a) | 37.8 ± 2.96 (a) | 38.8 ± 2.25 (a) |
| Total-cholesterol (mg/dL) | 98.3 ± 3.25 | 137.3 ± 3.28 (a) | 127.8 ± 4.88 (a) | 107.2 ± 4.33 (b,c) | 108.3 ± 3.13 (b,c) | 109.0 ± 2.97 (b,c) |
| HDL-cholesterol (mg/dL) | 79.3 ± 2.52 | 108.6 ± 2.43 (a) | 100.6 ± 2.33 (a) | 87.9 ± 4.00 (b,c) | 84.7 ± 4.21 (b,c) | 80.7 ± 3.84 (b,c) |
| ALT (UI/L) | 21.9 ± 1.27 | 32.9 ± 2.47 (a) | 31.5 ± 1.26 (a) | 20.5 ± 1.22 (b,c) | 20.3 ± 1.74 (b,c) | 20.6 ± 1.53 (b,c) |
| ALP (UI/L) | 41.7 ± 1.96 | 54.6 ± 3.49 (a) | 52.3 ± 1.74 (a) | 42.4 ± 2.60 (b,c) | 42.0 ± 2.75 (b,c) | 44.5 ± 1.22 (b,c) |
| Albumin (g/dL) | 2.13 ± 0.021 | 2.12 ± 0.028 | 2.14 ± 0.025 | 2.09 ± 0.043 | 2.08 ± 0.028 | 2.12 ± 0.027 |
| Creatinine (mg/dL) | 0.16 ± 0.003 | 0.15 ± 0.002 | 0.16 ± 0.004 | 0.15 ± 0.003 | 0.16 ± 0.004 | 0.15 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, B.P.; Pinheiro, C.G.; Figueiredo, I.D.; Cardoso, F.N.; Oliveira, J.O.; Machado, R.T.A.; da Silva, P.B.; Chorilli, M.; Brunetti, I.L.; Baviera, A.M. Combined Effects of Lycopene and Metformin on Decreasing Oxidative Stress by Triggering Endogenous Antioxidant Defenses in Diet-Induced Obese Mice. Molecules 2022, 27, 8503. https://doi.org/10.3390/molecules27238503
Motta BP, Pinheiro CG, Figueiredo ID, Cardoso FN, Oliveira JO, Machado RTA, da Silva PB, Chorilli M, Brunetti IL, Baviera AM. Combined Effects of Lycopene and Metformin on Decreasing Oxidative Stress by Triggering Endogenous Antioxidant Defenses in Diet-Induced Obese Mice. Molecules. 2022; 27(23):8503. https://doi.org/10.3390/molecules27238503
Chicago/Turabian StyleMotta, Bruno Pereira, Camila Graça Pinheiro, Ingrid Delbone Figueiredo, Felipe Nunes Cardoso, Juliana Oriel Oliveira, Rachel Temperani Amaral Machado, Patrícia Bento da Silva, Marlus Chorilli, Iguatemy Lourenço Brunetti, and Amanda Martins Baviera. 2022. "Combined Effects of Lycopene and Metformin on Decreasing Oxidative Stress by Triggering Endogenous Antioxidant Defenses in Diet-Induced Obese Mice" Molecules 27, no. 23: 8503. https://doi.org/10.3390/molecules27238503