Effect of Bentonite Addition to Pedro Ximénez White Grape Musts before Their Fermentation with Selected Yeasts on the Major Volatile Compounds and Polyols of Wines and Tentative Relationships with the Sensorial Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Winemaking Variables
2.2. Effects on the Major Volatile Compounds and Polyols
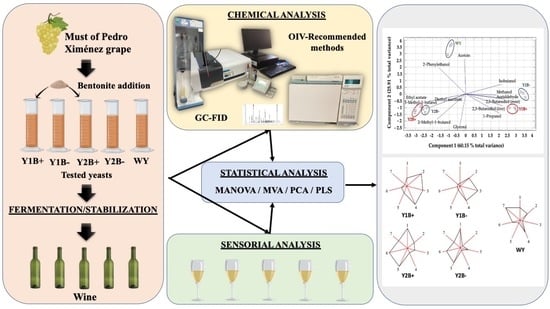
2.3. Statistical Analysis of the Data Matrix: Footprints and Principal Components Analysis
2.4. Sensorial Analysis of Wines
2.5. Tentative Correlations between Sensory Attributes and Chemical Variables
3. Materials and Methods
3.1. Wines and Winemaking Conditions
3.2. Analytical Methods
3.3. Sensorial Analysis
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Huang, L.; Ma, Y.; Tian, X.; Li, J.; Li, L.; Tang, K.; Xu, L. Chemosensory characteristics of regional Vidal icewines from China and Canada. Food Chem. 2018, 261, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Lambert-Royo, M.I.; Gil i Cortiella, M.; Del Barrio-Galán, R.; Peña-Neira, Á. Chemical, physical, and sensory effects of the use of bentonite at different stages of the production of traditional sparkling wines. Foods 2021, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Effect of bentonite fining on odor-active compounds in two different white wine styles. Am. J. Enol. Vitic. 2010, 61, 225–233. [Google Scholar] [CrossRef]
- He, S.; Hider, R.; Zhao, J.; Tian, B. Effect of bentonite fining on proteins and phenolic composition of Chardonnay and Sauvignon Blanc wines. S. Afr. J. Enol. Vitic. 2020, 41, 113–120. [Google Scholar] [CrossRef]
- Vela, E.; Hernández-Orte, P.; Castro, E.; Ferreira, V.; Lopez, R. Effect of bentonite fining on polyfunctional mercaptans and other volatile compounds in Sauvignon blanc wines. Am. J. Enol. Vitic. 2017, 68, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Condé, B.; Keenan, C.; Bodet, A.; Robinson, A.; Gonzalez-Viejo, C.; Xiao, P.; Fuentes, S.; Howell, K. Evaluation of the effect of bentonite fining for sparkling wine quality. Wine Vit. J. 2017, 32, 24–26. [Google Scholar]
- Catarino, S.; Madeira, M.; Monteiro, F.; Rocha, F.; Curvelo-Garcia, A.S.; De Sousa, R.B. Effect of bentonite characteristics on the elemental composition of wine. J. Agric. Food Chem. 2008, 56, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Van Sluyter, S.C.; McRae, J.M.; Falconer, R.J.; Smith, P.A.; Bacic, A.; Waters, E.J.; Marangon, M. Wine protein haze: Mechanisms of formation and advances in prevention. J. Agric. Food Chem. 2015, 63, 4020–4030. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.; Peinado, R.A. Wine Colloids. In Enological Chemistry; Elsevier: San Diego, CA, USA, 2012; pp. 346–347. [Google Scholar]
- Lira, E.; Salazar, F.N.; Rodríguez-Bencomo, J.J.; Vincenzi, S.; Curioni, A.; López, F. Effect of using bentonite during fermentation on protein stabilisation and sensory properties of white wine. Int. J. Food Sci. Technol. 2014, 49, 1070–1078. [Google Scholar] [CrossRef]
- Lira, E.; Rodríguez-Bencomo, J.J.; Salazar, F.N.; Orriols, I.; Fornos, D.; López, F. Impact of bentonite additions during vinification on protein stability and volatile compounds of Albariño wines. J. Agric. Food Chem. 2015, 63, 3004–3011. [Google Scholar] [CrossRef]
- Pocock, K.F.; Salazar, F.N.; Waters, E.J. The effect of bentonite fining at different stages of white winemaking on protein stability. Aust. J. Grape Wine Res. 2011, 17, 280–284. [Google Scholar] [CrossRef]
- Horvat, I.; Radeka, S.; Plavša, T.; Lukić, I. Bentonite fining during fermentation reduces the dosage required and exhibits significant side-effects on phenols, free and bound aromas, and sensory quality of white wine. Food Chem. 2019, 285, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ayestaran, B.M.; Ancin, M.C.; Garcia, A.M.; Gonzalez, A.; Garrido, J.J. Influence of prefermentation clarification on nitrogenous contents of musts and wines. J. Agric. Food Chem. 1995, 43, 476–482. [Google Scholar] [CrossRef]
- Puig-Deu, M.; López-Tamames, E.; Buxaderas, S.; Torre-Boronat, M.C. Quality of base and sparkling wines as influenced by the type of fining agent added pre-fermentation. Food Chem. 1999, 66, 35–42. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Comparing the impact of bentonite addition for both must clarification and wine fining on the chemical profile of wine from Chambave Muscat grapes. Int. J. Food Sci. Tech. 2012, 47, 1–12. [Google Scholar] [CrossRef]
- Somers, T.C.; Ziemelis, G. The use of gel column analysis in evaluation of bentonite fining procedures. Am. J. Enol. Vitic. 1973, 24, 51–54. [Google Scholar]
- Lisanti, M.T.; Gambuti, A.; Genovese, A.; Piombino, P.; Moio, L. Earthy off-flavour in wine: Evaluation of remedial treatments for geosmin contamination. Food Chem. 2014, 154, 171–178. [Google Scholar] [CrossRef]
- Alfonzo, A.; Prestianni, R.; Gaglio, R.; Matraxia, M.; Maggio, A.; Naselli, V.; Craparo, V.; Badalamenti, N.; Bruno, M.; Vagnoli, P.; et al. Effects of different yeast strains, nutrients and glutathione-rich inactivated yeast addition on the aroma characteristics of Catarratto wines. Int. J. Food Microbiol. 2021, 360, 109325. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Moio, L. Influence of yeast strain on odor-active compounds in fiano wine. Appl. Sci. 2021, 11, 7767. [Google Scholar] [CrossRef]
- Guarcello, R.; Gaglio, R.; Todaro, A.; Alfonzo, A.; Schicchi, R.; Cirlincione, F.; Moschetti, G.; Francesca, N. Insights into the cultivable microbial ecology of “manna” ash products extracted from Fraxinus angustifolia (Oleaceae) trees in Sicily, Italy. Front. Microbiol. 2019, 10, 984. [Google Scholar] [CrossRef]
- Blanco, P.; Vázquez-Alén, M.; Garde-Cerdán, T.; Vilanova, M. Application of autochthonous yeast Saccharomyces cerevisiae xg3 in treixadura wines from D.O. Ribeiro (nw spain): Effect on wine aroma. Fermentation 2021, 7, 31. [Google Scholar] [CrossRef]
- Duarte, F.L.; Baleiras-Couto, M.M. Survey of inoculated commercial Saccharomyces cerevisiae in winery-based trials. Fermentation 2021, 7, 176. [Google Scholar] [CrossRef]
- Tufariello, M.; Pati, S.; Palombi, L.; Grieco, F.; Losito, I. Use of Multivariate Statistics in the Processing of Data on Wine Volatile Compounds Obtained by HS-SPME-GC-MS. Foods 2022, 11, 910. [Google Scholar] [CrossRef]
- Estrada, M.A.R.; Park, D.; Chin, A.T.H. Measuring wine industry efficiency with wine industry network evaluation model (Wine-model). Contemp. Econ. 2020, 14, 272–284. [Google Scholar] [CrossRef]
- Xifang, S.; Chun, L.; Zhansheng, W.; Xiaolin, X.; Ling, R.; Hongsheng, Z. Adsorption of protein from model wine solution by different bentonites. Chin. J. Chem. Eng. 2007, 15, 632–638. [Google Scholar]
- Ma, T.Z.; Gong, P.F.; Lu, R.R.; Zhang, B.; Morata, A.; Han, S.Y. Effect of different clarification treatments on the volatile composition and aromatic attributes of ‘Italian Riesling’ Icewine. Molecules 2020, 25, 2657. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- García, M.J.; Aleixandre, J.L.; Álvarez, I.; Lizama, V. Foam aptitude of Bobal variety in white sparkling wine elaboration and study of volatile compounds. Eur. Food Res. Technol. 2009, 229, 133–139. [Google Scholar] [CrossRef]
- Armada, L.; Falqué, E. Repercussion of the clarification treatment agents before the alcoholic fermentation on volatile composition of white wines. Eur. Food Res. Technol. 2007, 225, 553–558. [Google Scholar] [CrossRef]
- Di Gaspero, M.; Ruzza, P.; Hussain, R.; Vincenzi, S.; Biondi, B.; Gazzola, D.; Siligardi, G.; Curioni, A. Spectroscopy reveals that ethyl esters interact with proteins in wine. Food Chem. 2017, 217, 373–378. [Google Scholar] [CrossRef]
- Sommer, S.; Tondini, F. Sustainable replacement strategies for bentonite in wine using alternative protein fining agents. Sustainability 2021, 13, 1860. [Google Scholar] [CrossRef]
- Vararu, F.; Moreno-Garcia, J.; Moreno, T.; Niculaua, M.; Nechita, B.; Zamfir, C.; Colibaba, C.; Dumitru, G.D.; Cotea, V.V. Minor volatile compounds profiles of ‘Aligoté’ wines fermented with different yeast strains. Not. Sci. Biol. 2015, 7, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Vararu, F.; Moreno-García, J.; Niculaua, M.; Cotea, V.V.; Mayén, M.; Moreno, J. Fermentative volatilome modulation of Muscat Ottonel wines by using yeast starter cultures. LWT-Food Sci. Technol. 2020, 129, 109575. [Google Scholar] [CrossRef]
- Vilanova, M.; Masneuf-Pomarède, I.; Dubourdieu, D. Influence of Saccharomyces cerevisiae strains on general composition and sensorial properties of white wines made from Vitis vinifera cv. Albariño. Food Technol. Biotechnol. 2005, 43, 79–83. [Google Scholar]
- Parish, K.J.; Herbst-Johnstone, M.; Bouda, F.; Klaere, S.; Fedrizzi, B. Pre-fermentation fining effects on the aroma chemistry of Marlborough Sauvignon blanc press fractions. Food Chem. 2016, 208, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of combined effect of proteins and bentonite fining on the wine aroma loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef]
- Casal del Rey, J. Análisis sensorial y cata de los vinos de España; Agrícola Española Madrid: Madrid, Spain, 2005. [Google Scholar]
- Brereton, R.G. (Ed.) Calibration. Appl. Chemom. Sci. 2007, 193–220. [Google Scholar]
- Dumitriu, G.D.; López de Lerma, N.; Luchian, C.E.; Cotea, V.V.; Peinado, R.A. Study of the potential use of mesoporous nanomaterials as fining agent to prevent protein haze in white wines and its impact in major volatile aroma compounds and polyols. Food Chem. 2018, 240, 751–758. [Google Scholar] [CrossRef]
- OIV. 2021. Available online: https://www.oiv.int/en (accessed on 1 May 2022).
- Peinado, R.A.; Moreno, J.A.; Muñoz, D.; Medina, M.; Moreno, J. Gas chromatographic quantification of major volatile compounds and polyols in wine by direct injection. J. Agr. Food Chem. 2004, 52, 6389–6393. [Google Scholar] [CrossRef]
- Martínez-García, R.; Mauricio, J.C.; García-Martínez, T.; Peinado, R.A.; Moreno, J. Towards a better understanding of the evolution of odour-active compounds and the aroma perception of sparkling wines during ageing. Food Chem. 2021, 357, 129784. [Google Scholar] [CrossRef] [PubMed]



| Y1B+ | Y1B− | Y2B+ | Y2B− | WY | HGs | |
|---|---|---|---|---|---|---|
| Ethanol (% v/v) | 11.8 ± 0.2 a | 12 ± 0.2 b | 12 ± 0.2 b | 12.5 ± 0.2 c | 11.9 ± 0.2 ab | 3 |
| pH | 3.43 ± 0.00 b | 3.49 ± 0.01 d | 3.22 ± 0.00 a | 3.22 ± 0.01 a | 3.46 ± 0.00 c | 4 |
| Volatile acidity (g L−1) | 0.34 ± 0.00 c | 0.40 ± 0.00 d | 0.24 ± 0.00 a | 0.24 ± 0.00 a | 0.26 ± 0.00 b | 4 |
| Total acidity (g L−1) | 3.97 ± 0.00 b | 3.81 ± 0.00 a | 6.94 ± 0.00 e | 6.60 ± 0.04 d | 4.04 ± 0.00 c | 5 |
| Reducing sugars (g L−1) | 1.92 ± 0.00 b | 2.28 ± 0.12 c | 1.68 ± 0.00 a | 2.64 ± 0.00 d | 3.12 ± 0.00 e | 5 |
| IPT | 7.26 ± 0.04 b | 8.48 ± 0.08 d | 7.41 ± 0.08 c | 7.53 ± 0.07 c | 6.93 ± 0.09 a | 4 |
| Absorbance 420 nm | 0.1934 ± 0.0005 c | 0.1656 ± 0.0004 b | 0.1935 ± 0.0003 c | 0.2170 ± 0.0009 d | 0.1635 ± 0.0007 a | 4 |
| Absorbance 520 nm | 0.0757 ± 0.0003 d | 0.0403 ± 0.0001 a | 0.0512 ± 0.0005 c | 0.0747 ± 0.0012 d | 0.0436 ± 0.0003 b | 4 |
| Absorbance 620 nm | 0.0483 ± 0.0005 d | 0.0114 ± 0.0005 a | 0.0154 ± 0.0006 b | 0.038 ± 0.002 c | 0.0166 ± 0.0002 b | 4 |
| Compounds | PubChem CID | Y1B+ | Y1B− | Y2B+ | Y2B− | WY | HG |
|---|---|---|---|---|---|---|---|
| Acetaldehyde | 177 | 106 ± 8 c | 161 ± 4 d | 40 ± 2 a | 63 ± 1 b | 58 ± 5 b | 4 |
| Ethyl acetate | 8857 | 10.1 ± 0.3 b | 7.6 ± 0.9 a | 15.9 ± 0.6 d | 15.2 ± 0.6 d | 12.2 ± 0.3 c | 4 |
| Methanol | 137654 | 109 ± 8 d | 87 ± 3 c | 76 ± 2 b | 55 ± 2 a | 78 ± 3 b | 4 |
| Propanol | 1031 | 54 ± 1 c | 59.1 ± 0.5 d | 39 ± 1 b | 39.5 ± 0.8 b | 29.7 ± 0.9 a | 4 |
| Isobutanol | 6560 | 47.1 ± 0.4 e | 44.1 ± 0.5 d | 35.7 ± 0.3 b | 33.9 ± 0.4 a | 42.1 ± 0.3 c | 5 |
| 2-Methyl-1-butanol | 8723 | 44 ± 1 c | 38.5 ± 0.7 b | 65 ± 3 e | 59.6 ± 0.3 d | 36.4 ± 0.4 a | 5 |
| 3-Methyl-1-butanol | 31260 | 178 ± 2 b | 163 ± 2 a | 236 ± 7 e | 217 ± 3 d | 186 ± 1 c | 5 |
| Acetoin | 173 | 9 ± 2 a | 14 ± 1 c | 11 ± 1 bc | 9 ± 1 a | 20 ± 2 d | 4 |
| 2,3-Butanediol levo | 225936 | 749 ± 56 b | 714 ± 29 b | 319 ± 36 a | 354 ± 25 a | 303 ± 72 a | 2 |
| 2,3-Butanediol meso | 220010 | 265 ± 17 b | 271 ± 24 b | 111 ± 12 a | 127 ± 9 a | 142 ± 32 a | 2 |
| Diethyl succinate | 31249 | 21.6 ± 0.9 a | 21 ± 1 a | 23 ± 2 a | 22 ± 1 a | 21 ± 2 a | 1 |
| 2-Phenylethanol | 7409 | 30 ± 3 a | 31.1 ± 0.7 a | 56 ± 3 c | 48 ± 5 b | 79 ± 5 d | 4 |
| Glycerol (g L−1) | 753 | 11.9 ± 0.4 c | 10.8 ± 0.5 b | 12.7 ± 0.9 d | 12.0 ± 0.4 c | 8.6 ± 0.9 a | 4 |
| Yeast | Y1B+ | Y1B− | Y2B+ | Y2B− | WY | HGs | ANOVA (p Values) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attributes | Mean | STD | Mean | STD | Mean | STD | Mean | STD | Mean | STD | Yeast | Bentonite | |
| Sight | 8.88 c | 0.60 | 8.88 c | 0.78 | 7.88 b | 0.60 | 7.38 ab | 0.70 | 6.75 a | 0.66 | 3 | 0.000 | 0.436 |
| Smell | 13.75 ab | 0.97 | 14.63 bc | 1.32 | 15.50 c | 1.32 | 15.00 bc | 1.58 | 12.38 a | 1.11 | 3 | 0.001 | 0.758 |
| Taste | 30.25 a | 4.18 | 30.38 a | 3.81 | 28.13 a | 3.02 | 28.88 a | 3.10 | 27.38 a | 3.94 | 1 | 0.140 | 0.662 |
| Overall quality | 23.00 b | 2.35 | 23.13 b | 3.14 | 21.38 ab | 2.12 | 21.75 ab | 2.44 | 19.63 a | 1.11 | 2 | 0.018 | 0.804 |
| Total points | 75.88 b | 6.47 | 77.00 b | 6.40 | 72.88 ab | 6.55 | 73.00 b | 6.36 | 66.13 a | 5.60 | 2 | 0.005 | 0.850 |
| PLS for TASTE | PLS for Sight | PLS for SMELL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LV 1 | LV 2 | LV 3 | LV 1 | LV 2 | LV 1 | LV 2 | |||
| Taste Qual | 0.671 | 0.263 | 0.145 | Sight overall | 0.726 | 0.189 | Smell Qual | 0.398 | 0.39 |
| % variance | 45.16 | 84.01 | 99.89 | % variance | 17.63 | 65.45 | % variance | 38.03 | 72.68 |
| p-value | 0.00013 | p-value | 0.022 | p-value | 0.029 | ||||
| RMSE | 0.2 | RMSE | 0.133 | RMSE | 0.133 | ||||
| Independent Variables and Latent Variables Selected | |||||||||
| Variables | LV 1 | LV 2 | LV 3 | Variables | LV 1 | LV 2 | Variables | LV 1 | LV 2 |
| pH | 0.158 | -0.558 | 0.149 | pH | 0.115 | −0.428 | Acetic acid | −0.023 | 0.242 |
| Acetic acid | 0.531 | 0.029 | 0.629 | Ethanol | −0.3 | −0.414 | Acetaldehyde | −0.093 | 0.118 |
| Ethanol | −0.399 | 0.374 | 0.762 | Abs (420) | 0.101 | 0.507 | Ethyl acetate | 0.157 | −0.116 |
| Reducing sugars | −0.73 | −0.74 | −0.034 | Abs (520) | 0.113 | 0.43 | 1-Propanol | 0.192 | 0.379 |
| Abs (620) | 0.092 | 0.313 | Isobutanol | 0.071 | 0.392 | ||||
| Abs (280) | 0.93 | 0.325 | 3-Methyl-1-butanol | 0.313 | 0.071 | ||||
| 2-Methyl-1-butanol | 0.246 | −0.002 | |||||||
| Acetoin | −0.077 | 0.049 | |||||||
| Diethyl succinate | 0.871 | 0.771 | |||||||
| 2-Phenylethanol | −0.034 | −0.125 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Castells, R.; Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Effect of Bentonite Addition to Pedro Ximénez White Grape Musts before Their Fermentation with Selected Yeasts on the Major Volatile Compounds and Polyols of Wines and Tentative Relationships with the Sensorial Evaluation. Molecules 2022, 27, 8057. https://doi.org/10.3390/molecules27228057
Muñoz-Castells R, Moreno-García J, García-Martínez T, Mauricio JC, Moreno J. Effect of Bentonite Addition to Pedro Ximénez White Grape Musts before Their Fermentation with Selected Yeasts on the Major Volatile Compounds and Polyols of Wines and Tentative Relationships with the Sensorial Evaluation. Molecules. 2022; 27(22):8057. https://doi.org/10.3390/molecules27228057
Chicago/Turabian StyleMuñoz-Castells, Raquel, Jaime Moreno-García, Teresa García-Martínez, Juan Carlos Mauricio, and Juan Moreno. 2022. "Effect of Bentonite Addition to Pedro Ximénez White Grape Musts before Their Fermentation with Selected Yeasts on the Major Volatile Compounds and Polyols of Wines and Tentative Relationships with the Sensorial Evaluation" Molecules 27, no. 22: 8057. https://doi.org/10.3390/molecules27228057