A Review on the Synthesis and Chemical Transformation of Quinazoline 3-Oxides
Abstract
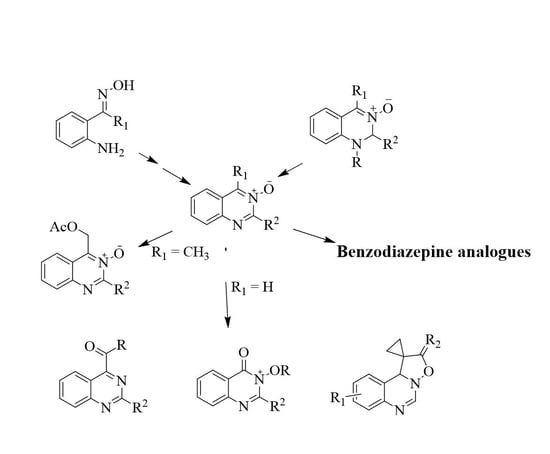
:1. Introduction
2. Methods for the Synthesis of Quinazoline Oxides
2.1. Direct Oxidation of Quinazolines
2.2. Synthesis of Quinazoline 3-Oxides
2.2.1. Intramolecular Cyclocondensation of the N-Acyl-2-aminoaryl Ketone Oximes
2.2.2. Transition Metal-Mediated Reactions to Afford Quinazoline N-Oxides
2.3. Dehydrogenation of the 1,2-Dihydroquinazoline 3-Oxides
2.4. Chemical Transformation of Quinazoline 3-Oxides
2.4.1. Deoxygenation of Quinazoline N-Oxides
2.4.2. Alkoxylation of Quinazoline N-Oxides
2.4.3. Alkylation of Quinazoline N-Oxides
2.5. Synthesis of Polycyclic Quinazoline Derivatives and Benzodiazepine Analogues
2.6. Ring Expansion of Quinazoline 3-Oxides to Afford Benzodiazepine Analogues
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yerragunta, V.; Patil, P.; Anusha, V.; KumaraSwamy, T.; Suman, S.; Samhitha, T. Pyrimidine and its biological activity: A review. PharmaTutor 2013, 1, 39–44. [Google Scholar]
- Kočevar, M.; Kokvar, W.; Mlakar, B.; Perdič, M.; Petrie, A.; Polaac, B.; Verček, B. The synthesis of pyrimidine l-oxides: A new transformation of amide oxides. Tetrahedron Lett. 1992, 33, 2195–2198. [Google Scholar] [CrossRef]
- Ashburn, S.P.; Coates, R.M. Preparation of oxazoline N-oxides and imidate N-oxides by amide acetal condensation and their [3+2] cycloaddition reaction. J. Org. Chem. 1985, 50, 3073–3076. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Chitra, K.; Saravanan, G.; Solomon, V.R.; Sulthana, M.T.; Narendhar, B. An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 2018, 151, 628–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, F. Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 2013, 7, 95. [Google Scholar] [CrossRef] [Green Version]
- Faheem, M.; Tiwan, A.K.; Singh, V.K. A review on modern synthetic route for the construction of 1,3-diazanaphthalene moiety. Curr. Org. Chem. 2020, 24, 1108–1138. [Google Scholar] [CrossRef]
- Faisal, M.; Saeed, A. Chemical insights into the synthetic chemistry of quinazolines: Recent advances. Front. Chem. 2021, 8, 594717. [Google Scholar] [CrossRef]
- Ye, X.; Chen, Z.; Zhang, Z.; Fu, Y.; Deng, Z.; Peng, Y. A convenient approach to 2,4-disubstituted quinazoline-3-oxides using active MnO2 as the oxidant. Can. J. Chem. 2019, 97, 682–689. [Google Scholar] [CrossRef]
- Yin, Z.; Li, X.; Deng, Z.; Yang, Q.; Peng, Y. The synthesis of isoxazolo[2,3-c]quinazolines via a cycloaddition of quinazoline-3-oxides and acrylates. Tetrahedron Lett. 2020, 61, 151818. [Google Scholar] [CrossRef]
- Pathare, R.S.; Maurya, A.K.; Kumari, A.; Agnihotri, V.K.; Vermac, V.P.; Sawant, D.M. Synthesis of quinazoline-3-oxides via a Pd(II) catalyzed azide–isocyanide coupling/cyclocondensation reaction. Org. Biomol. Chem. 2019, 17, 363–368. [Google Scholar] [CrossRef]
- Higashino, T.; Amano, T.; Tamura, Y.; Katsumata, N.; Washizu, Y.; Takeshi, O.; Hayashi, E. On N-oxidation of 4-alkyl-, 4-phenyl-quinazoline and reaction of 4-methylquinazoline-1-oxide. Chem. Pharm. Bull. 1972, 20, 1874–1882. [Google Scholar] [CrossRef]
- Petkevičius, V.; Vaitekũnas, J.; Tauraite, D.; Stankevičiũtė, J.; Šarlauskas, J.; Čėnas, J.; Meškys, R. A biocatalytic synthesis of heteroaromaticn-oxides by whole cells of Escherichia coli expressing the multicomponent, soluble di-iron monooxygenase (SDIMO) PmlABCDEF. Adv. Synth. Catal. 2019, 361, 2456–2465. [Google Scholar]
- Armarego, W.L.F. Quinazolines. Part V. Covalent hydration in quinazoline 3-oxides. J. Chem. Soc. 1962, 5030–5036. [Google Scholar] [CrossRef]
- Kovendi, A.; Kircz, M. New synthesis for quinazoline N3-oxides and 1,2-dihydroquinazoline N3-oxides. Chem. Ber. 1965, 9, 1049–1059. [Google Scholar]
- Alzogaray, R.A.; Fontán, A.; Camps, F.; Masuh, H.; Orihuela, P.S.; Fernández, D.; Cork, A.; Zerba, E. Behavioural response of Triatoma infestans (Klug) (Hemiptera: Reduviidae) to quinazolines. Molecules 2005, 10, 1190–1196. [Google Scholar] [CrossRef]
- Heaney, F.; Lawless, E. 2-Vinyl quinazoline 3-oxide; preparation from acid induced cyclocondensation of 2-acylaminoaryloximes. J. Heterocycl. Chem. 2007, 44, 569–574. [Google Scholar] [CrossRef] [Green Version]
- Combs, D.W.; Rampulla, M.S.; Russell, R.K.; Rampulla, R.; Klaubert, D.H.; Ritchie, D.; Meeks, A.S.; Kirchner, T. Design, synthesis and bronchodilatory activity of a series of quinazoline-3-oxides. Drug Des. Del. 1990, 6, 241–254. [Google Scholar]
- Combs, D.W.; Fallotico, R. Substituted Quinazoline-3-Oxides Providing Pharmacological Activity. U.S. Patent 4,745,118, 17 May 1988. [Google Scholar]
- Samandram, R.; Korukçu, M.Ç.; Coşkun, N. Eco-friendly H2O2 oxidation of 1,2-dihydroquinazoline-3-oxides to quinazoline-3-oxides. Synth. Commun. 2021, 51, 2349–2356. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Onwu, E.E.; Agbo, E.N.; Maluleka, M.M.; More, G.K.; Choong, Y.S. Synthesis, in vitro and in silico enzyme (COX-1/2 & LOX-5), free radical scavenging profiling of the 2,4-dicarbo substituted quinazoline 3-oxides. Med. Chem. Res. 2022, 31, 146–164. [Google Scholar]
- Jatangi, N.; Palakodety, R.K. I2-Catalyzed oxidative synthesis of N,4-disubstituted quinazolines and quinazoline oxides. Org. Biomol. Chem. 2019, 17, 3714–3717. [Google Scholar] [CrossRef]
- Counceller, C.M.; Eichman, C.C.; Wray, B.C.; Stambuli, J.P. A practical, metal-free synthesis of 1H-indazoles. Org. Lett. 2008, 10, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, M.J.; Magwaza, N.M.; Gildenhuys, S.; Setshedi, I.B. Synthesis, α-glucosidase inhibition and antioxidant activity of the 7-carbo–substituted 5-bromo-3-methylindazoles. Bioorg. Chem. 2020, 97, 103702. [Google Scholar] [CrossRef] [PubMed]
- Wray, B.C.; Stambuli, J.P. Synthesis of N-arylindazoles and benzimidazoles from a common intermediate. Org. Lett. 2010, 12, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Renaut, P.P.; Durand, P.; Ratel, P. 3,9-Dihydro-2H-[1,2,4]-oxadiazolo[3,2-b]quinazolin-2-ones: First synthesis of the parent heterocycle, 7- and 9-substituted derivatives. Synthesis 2000, 14, 2009–2012. [Google Scholar] [CrossRef]
- Madabhushi, S.; Mallu, K.K.R.; Jillella, R.; Kurva, S.; Singh, R. One-step method for synthesis of 2,4-disubstituted quinazoline 3-oxides by reaction of a 2-aminoaryl ketone with a hydroxamic acid using Zn(OTf)2 as the catalyst. Tetrahedron Lett. 2014, 55, 1979–1982. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Yang, X.; Zhou, X.; Li, X. Rh(III)- and Zn(II)-catalyzed synthesis of quinazoline N-oxides via C–H amidation–cyclization of oximes. Org. Lett. 2016, 18, 6144–6147. [Google Scholar] [CrossRef]
- Heaney, F.; McCarthy, T.; Mahon, M.; Nacereddine, V. Bridgehead nitrogen heterocycles which contain the quinazoline moiety–synthesis and cycloaddition of 1,2-dihydroquinazoline 3-oxides. Org. Biomol. Chem. 2005, 3, 4351–4361. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Yang, D.-Y. Visible light-mediated synthesis of quinazolines from 1,2-dihydroquinazoline-3-oxides. Tetrahedron 2013, 69, 10438–10444. [Google Scholar] [CrossRef]
- Mikiciuk-Olasik, E.; Baszczak-Światkiewiz, K.; Żurek, E.; Krajewska, U.; Różalski, M.; Kruszyński, R.; Bartczak, T.J. New derivatives of quinazoline and 1,2-dihydroquinazoline N3-oxide with expected antitumor activity. Arch. Pharm. Pharm. Med. Chem. 2004, 337, 239–246. [Google Scholar] [CrossRef]
- Wu, C.-K.; Yang, D.-Y. Visible-light-mediated reaction: Synthesis of quinazolinones from 1,2-dihydroquinazoline 3-oxides. RSC Adv. 2016, 6, 65988–65994. [Google Scholar] [CrossRef]
- Eynde, J.J.V.; Godin, J.; Mayence, A.; Maquestiau, A.; Anders, E. A new and convenient method for the preparation of 2-substituted quinazolines. Synthesis 1993, 9, 867–869. [Google Scholar] [CrossRef]
- Coșkun, N.; Cetin, M. A new regioselective synthesis and ambient light photochemistry of quinazoline 1-oxides. Tetrahedron 2007, 63, 2966–2972. [Google Scholar] [CrossRef]
- Fan, L.; Wang, T.; Tian, Y.; Xiong, F.; Wu, S.; Liang, Q.; Zhao, J. Copper-catalyzed oxidative coupling between quinazoline 3-oxides and unactivated aldehydes: An efficient approach to functionalized quinazolines. Chem. Commun. 2016, 52, 5375–5378. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, Z.; Wang, T.; Liang, Q.; Zhao, J. Copper-catalyzed oxidative functionalization of benzylic C–H bonds with quinazoline 3-oxides. Org. Chem. Front. 2018, 5, 2492–2495. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Y.; Yu, W.; Fan, L.; Wang, T.; Fu, J. Copper-catalyzed oxidative coupling of quinazolin-3-oxides: Synthesis of O-quinazolinic carbamates. J. Org. Chem. 2022, 87, 5136–5148. [Google Scholar] [CrossRef]
- Luo, J.; Wan, J.; Wu, L.; Yang, L.; Wang, T. tert-Butyl hydroperoxide promoted the reaction of quinazoline-3-oxides with primary amines affording quinazolin-4(3H)-ones. J. Org. Chem. 2022, 87, 9864–9874. [Google Scholar] [CrossRef]
- Yang, Q.; Lou, M.; Yin, Z.; Deng, Z.; Ding, Q.; Peng, Y. Direct C-4 alkylation of quinazoline N-oxides with ethers via an oxidative cross-coupling reaction under metal-free conditions. Org. Biomol. Chem. 2018, 45, 8724–8731. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, Z.; Zheng, L.; Yuan, J.; Wei, S.; Ding, Q.; Peng, Y. Copper-catalyzed cross-dehydrogenative coupling between quinazoline 3-oxides and indoles. RSC Adv. 2019, 9, 5870–5877. [Google Scholar] [CrossRef] [Green Version]
- Lüth, A.; Lowe, W. Syntheses of 4-(indole-3-yl)quinazolines—A new class of epidermal growth factor receptor tyrosine kinase (EGFR-TK) inhibitors. Eur. J. Med. Chem. 2008, 43, 1478–1488. [Google Scholar] [CrossRef]
- Lüth, A.; Lowe, W. A novel synthesis of EGFR-tyrosine-kinase inhibitors with 4-(indol-3-yl)quinazoline structure. J. Heterocycl. Chem. 2008, 45, 703–708. [Google Scholar] [CrossRef]
- An, Y.; Zheng, D.; Wu, J. An unexpected copper(ii)-catalyzed three-component reaction of quinazoline 3-oxide, alkylidenecyclopropane, and water. Chem. Commun. 2014, 50, 9165–9167. [Google Scholar] [CrossRef] [PubMed]
- Nacereddine, A.K. A MEDT computational study of the mechanism, reactivity and selectivity of non-polar [3+2] cycloaddition between quinazoline-3-oxide and methyl 3-methoxyacrylate. J. Mol. Model. 2020, 26, 328. [Google Scholar] [CrossRef] [PubMed]
- Heaney, J.F.; Lawless, E.; Mahon, M.; McArdle, P.; Cunningham, D. 1,3-Dipolar character of 2-vinyl quinazoline 3-oxides; first and second generation cycloaddition products. Org. Biomol. Chem. 2006, 4, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Kumar, N.; Anshu, A.; Sharma, P.; Kishore, D. 1,5-Benzodiazepines: Overview of properties and synthetic aspects. Res. J. Chem. Sci. 2013, 3, 90–103. [Google Scholar]





































| 39a–c | R1 | R2 | % Yield |
|---|---|---|---|
| 39a | H |  | 70 |
| 39b | H |  | 75 |
| 39c | CH3 |  | 60 |
| 81a–d | R1 | R2 | % Yield (Purification) |
|---|---|---|---|
| 81a | Ph | -CO2Me | 36 (SiO2); 30 (Al2O) |
| 81b | Me | -CO2Me | 34 (SiO2); 32 (Al2O) |
| 81c | Ph | H | 23 (SiO2); 60 (Al2O) |
| 81d | Me | H | 7 (SiO2); 54 (Al2O) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mphahlele, M.J. A Review on the Synthesis and Chemical Transformation of Quinazoline 3-Oxides. Molecules 2022, 27, 7985. https://doi.org/10.3390/molecules27227985
Mphahlele MJ. A Review on the Synthesis and Chemical Transformation of Quinazoline 3-Oxides. Molecules. 2022; 27(22):7985. https://doi.org/10.3390/molecules27227985
Chicago/Turabian StyleMphahlele, Malose J. 2022. "A Review on the Synthesis and Chemical Transformation of Quinazoline 3-Oxides" Molecules 27, no. 22: 7985. https://doi.org/10.3390/molecules27227985