Standardized Pomegranate (Pomella®) and Red Maple (Maplifa®) Extracts and Their Phenolics Protect Type I Collagen by the Inhibition of Matrix Metalloproteinases, Collagenase, and Collagen Cross-Linking
Abstract
:1. Introduction
2. Results
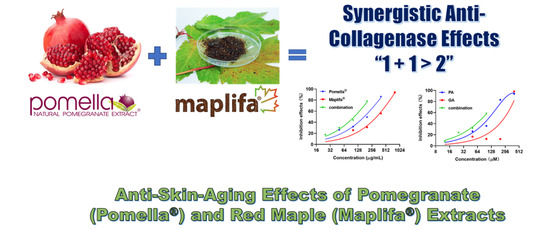
2.1. Pomella® and Maplifa® Inhibit Collagenase Activity and Their Combination Exerts Synergistic Effects
2.2. Punicalagin (PA) and Ginnalin A (GA) Interact with Collagenase Protein
2.3. Pomella®, Maplifa®, PA and GA Reduce Glycation-Induced Collagen Cross-Linking
2.4. Pomella®, Maplifa®, PA and GA Modulated Cell Viability in Cultured Normal Human Immortalized Keratinocytes (N/TERT1) and Epidermoid Carcinoma (A431) Cell Lines at Higher Concentrations
2.5. Pomella®, Maplifa®, PA and GA Reduced the Protein Expression of MMPs in Cultured N/TERT1 and A431 Cell Lines
2.6. Pomella®, Maplifa®, PA and GA Modulate the Protein Expression Levels of Type I Collagen and MMP-9 in Cultured Human Epidermoid Carcinoma A431 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals, Antibodies, and Reagents
4.2. Enzyme Inhibition Kinetic Assay
4.3. Cell Lines, Cell Cultures, Cytotoxicity and Viability Assay
4.4. Molecular Docking Analysis
4.5. Collagen Cross-Linking Inhibition Assay
4.6. Preparation of Cell Lysates, Western Blot Assay and Analysis
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonamonte, D.; Filoni, A.; De Marco, A.; Lospalluti, L.; Nacchiero, E.; Ronghi, V.; Colagrande, A.; Giudice, G.; Cazzato, G. Squamous Cell Carcinoma in Patients with Inherited Epidermolysis Bullosa: Review of Current Literature. Cells 2022, 11, 1365. [Google Scholar] [CrossRef] [PubMed]
- Nyström, A.; Bruckner-Tuderman, L.; Kiritsi, D. Dystrophic Epidermolysis Bullosa: Secondary Disease Mechanisms and Disease Modifiers. Front. Genet. 2021, 12, 737272. [Google Scholar] [CrossRef]
- Nowotny, K.; Grune, T. Degradation of Oxidized and Glycoxidized Collagen: Role of Collagen Cross-Linking. Arch. Biochem. Biophys. 2014, 542, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-Degrading Metalloproteinases in Photoaging. In Proceedings of the Journal of Investigative Dermatology Symposium Proceedings; Nature Publishing Group: London, UK, 2009; Volume 14, pp. 20–24. [Google Scholar]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Ghimeray, A.K.; Jung, U.S.; Lee, H.Y.; Kim, Y.H.; Ryu, E.K.; Chang, M.S. In Vitro Antioxidant, Collagenase Inhibition, and in Vivo Anti-Wrinkle Effects of Combined Formulation Containing Punica granatum, Ginkgo Biloba, Ficus Carica, and Morus Alba Fruits Extract. Clin. Cosmet. Investig. Dermatol. 2015, 8, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.N.; Lansky, E.P.; Varani, J. Pomegranate as a Cosmeceutical Source: Pomegranate Fractions Promote Proliferation and Procollagen Synthesis and Inhibit Matrix Metalloproteinase-1 Production in Human Skin Cells. J. Ethnopharmacol. 2006, 103, 311–318. [Google Scholar] [CrossRef]
- Kang, S.J.; Choi, B.R.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Song, C.H.; Ku, S.K.; Lee, Y.J. Beneficial Effects of Dried Pomegranate Juice Concentrated Powder on Ultraviolet B-Induced Skin Photoaging in Hairless Mice. Exp. Ther. Med. 2017, 14, 1023–1036. [Google Scholar] [CrossRef] [Green Version]
- Lisbeth, A.; Noratto, G.; Hingorani, L.; Talcott, S.T.; Mertens-Talcott, S.U. Protective Effects of Standardized Pomegranate (Punica granatum L.) Polyphenolic Extract in Ultraviolet-Irradiated Human Skin Fibroblasts. J. Agric. Food Chem. 2008, 56, 8434–8441. [Google Scholar] [CrossRef]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective Effect of Pomegranate-Derived Products on UVB-Mediated Damage in Human Reconstituted Skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef]
- Jin, Y.-J.; Ji, Y.; Jang, Y.-P.; Choung, S.-Y. Acer Tataricum Subsp. ginnala Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-ΚB, and TGFβ/Smad Signaling in UVB-Irradiated Human Dermal Fibroblasts. Molecules 2021, 26, 662. [Google Scholar] [CrossRef] [PubMed]
- Ginnalin B Induces Differentiation Markers and Modulates the Proliferation/Differentiation Balance via the Upregulation of NOTCH1 in Human Epidermal Keratinocytes. ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0968089619304389?via%3Dihub (accessed on 5 November 2022).
- Liu, C.; Guo, H.; Dain, J.A.; Wan, Y.; Gao, X.H.; Chen, H.D.; Seeram, N.P.; Ma, H. Cytoprotective Effects of a Proprietary Red Maple Leaf Extract and Its Major Polyphenol, Ginnalin A, against Hydrogen Peroxide and Methylglyoxal Induced Oxidative Stress in Human Keratinocytes. Food Funct. 2020, 11, 5105–5114. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X.H.; Chen, H.D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) Phenolics Ameliorate Hydrogen Peroxide-Induced Oxidative Stress and Cytotoxicity in Human Keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, U.; Schönauer, E.; Nüss, D.; Brandstetter, H. Structure of Collagenase G Reveals a Chew-and-Digest Mechanism of Bacterial Collagenolysis. Nat. Struct. Mol. Biol. 2010, 18, 1109–1114. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Seeram, N.P. Liquid Chromatography Coupled with Time-of-Flight Tandem Mass Spectrometry for Comprehensive Phenolic Characterization of Pomegranate Fruit and Flower Extracts Used as Ingredients in Botanical Dietary Supplements. J. Sep. Sci. 2018, 41, 3022–3033. [Google Scholar] [CrossRef]
- Li, C.; Seeram, N.P. Ultra-Fast Liquid Chromatography Coupled with Electrospray Ionization Time-of-Flight Mass Spectrometry for the Rapid Phenolic Profiling of Red Maple (Acer rubrum) Leaves. J. Sep. Sci. 2018, 41, 2331–2346. [Google Scholar] [CrossRef]
- Liu, W.; Ma, H.; Frost, L.; Yuan, T.; Dain, J.A.; Seeram, N.P. Pomegranate Phenolics Inhibit Formation of Advanced Glycation Endproducts by Scavenging Reactive Carbonyl Species. Food Funct. 2014, 5, 2996–3004. [Google Scholar] [CrossRef]
- Ma, H.; Liu, W.; Frost, L.; Kirschenbaum, L.J.; Dain, J.A.; Seeram, N.P. Glucitol-Core Containing Gallotannins Inhibit the Formation of Advanced Glycation End-Products Mediated by Their Antioxidant Potential. Food Funct. 2016, 7, 2213–2222. [Google Scholar] [CrossRef]
- Jean-Gilles, D.; Li, L.; Vaidyanathan, V.G.; King, R.; Cho, B.; Worthen, D.R.; Chichester, C.O.; Seeram, N.P. Inhibitory Effects of Polyphenol Punicalagin on Type-II Collagen Degradation in Vitro and Inflammation in Vivo. Chem. -Biol. Interact. 2013, 205, 90–99. [Google Scholar] [CrossRef]
- Ma, H.; Xu, J.; DaSilva, N.A.; Wang, L.; Wei, Z.; Guo, L.; Johnson, S.L.; Lu, W.; Xu, J.; Gu, Q.; et al. Cosmetic Applications of Glucitol-Core Containing Gallotannins from a Proprietary Phenolic-Enriched Red Maple (Acer rubrum) Leaves Extract: Inhibition of Melanogenesis via down-Regulation of Tyrosinase and Melanogenic Gene Expression in B16F10 Melanoma Ce. Arch. Dermatol. Res. 2017, 309, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, L.; Niesen, D.B.; Cai, A.; Cho, B.P.; Tan, W.; Gu, Q.; Xu, J.; Seeram, N.P. Structure Activity Related, Mechanistic, and Modeling Studies of Gallotannins Containing a Glucitol-Core and α-Glucosidase. RSC Adv. 2015, 5, 107904–107915. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.P.H.; Niehues, H.; Rikken, G.; van Vlijmen-Willems, I.M.J.J.; van de Zande, G.W.H.J.F.; Zeeuwen, P.L.J.M.; Schalkwijk, J.; van den Bogaard, E.H. Immortalized N/TERT Keratinocytes as an Alternative Cell Source in 3D Human Epidermal Models. Sci. Rep. 2017, 7, 11838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rikken, G.; Niehues, H.; van den Bogaard, E.H. Organotypic 3D Skin Models: Human Epidermal Equivalent Cultures from Primary Keratinocytes and Immortalized Keratinocyte Cell Lines. Methods Mol. Biol. 2020, 2154, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Boateng, S.T.; Banang-Mbeumi, S.; Singh, P.K.; Basnet, P.; Chamcheu, R.-C.N.; Ladu, F.; Chauvin, I.; Spiegelman, V.S.; Hill, R.A.; et al. Synthesis, Inverse Docking-Assisted Identification and in Vitro Biological Characterization of Flavonol-Based Analogs of Fisetin as c-Kit, CDK2 and MTOR Inhibitors against Melanoma and Non-Melanoma Skin Cancers. Bioorg. Chem. 2021, 107, 104595. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, E. Cross-Linking and Fluorescence Changes of Collagen by Glycation and Oxidation. Biochim. Biophys. Acta 1989, 998, 105–110. [Google Scholar] [CrossRef]
- Cervantes-Laurean, D.; Schramm, D.D.; Jacobson, E.L.; Halaweish, I.; Bruckner, G.G.; Boissonneault, G.A. Inhibition of Advanced Glycation End Product Formation on Collagen by Rutin and Its Metabolites. J. Nutr. Biochem. 2006, 17, 531–540. [Google Scholar] [CrossRef]
- Roy, T.; Boateng, S.T.; Banang-Mbeumi, S.; Singh, P.K.; Basnet, P.; Chamcheu, R.C.N.; Ladu, F.; Chauvin, I.; Spiegelman, V.S.; Hill, R.A.; et al. Identification of new fisetin analogs as kinase inhibitors: Data on synthesis and anti-skin cancer activities evaluation. Data Brief. 2021, 35, 106858. [Google Scholar] [CrossRef]







| Pomella® + Maplifa® (µg/mL) | Combination Index (CI) | Synergistic Inhibition | PA + GA (µM) | Combination Index (CI) | Synergistic Inhibition a |
|---|---|---|---|---|---|
| 12.5 + 25 | 0.53 | + | 12.5 + 12.5 | 0.87 | + |
| 25 + 50 | 0.67 | + | 25 + 25 | 0.84 | + |
| 50 + 100 | 0.90 | + | 50 + 50 | 1.34 | − |
| 100 + 200 | 0.70 | + | 100 + 100 | 1.47 | − |
| Compound | Concentration (µM) | Km (mM) | Vmax (mM/min) | Inhibition Type |
|---|---|---|---|---|
| PA | 0 | 7.743 | 0.191 | Uncompetitive |
| 100 | 1.425 | 0.035 | ||
| 400 | 1.006 | 0.025 | ||
| GA | 0 | 2.890 | 0.074 | Competitive |
| 100 | 4.027 | 0.078 | ||
| 400 | 4.957 | 0.078 |
| Sample | IC50 Values | |
|---|---|---|
| NTERT1 | A431 | |
| Maplifa® (µg/mL) | 160.6 ± 11.46 | 99.2 ± 0.6 |
| Pomella® (µg/mL) | 26.6 ± 5.27 | 38.8 ± 1.3 |
| PA (µM) | 46.1 ± 5.68 | 30.5 ± 1.0 |
| GA (µM) | 99.8 ± 3.75 | 49.5 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Roy, T.; Boateng, S.T.; He, H.; Liu, C.; Liu, W.; Li, D.; Wu, P.; Seeram, N.P.; Chamcheu, J.C.; et al. Standardized Pomegranate (Pomella®) and Red Maple (Maplifa®) Extracts and Their Phenolics Protect Type I Collagen by the Inhibition of Matrix Metalloproteinases, Collagenase, and Collagen Cross-Linking. Molecules 2022, 27, 7919. https://doi.org/10.3390/molecules27227919
Li H, Roy T, Boateng ST, He H, Liu C, Liu W, Li D, Wu P, Seeram NP, Chamcheu JC, et al. Standardized Pomegranate (Pomella®) and Red Maple (Maplifa®) Extracts and Their Phenolics Protect Type I Collagen by the Inhibition of Matrix Metalloproteinases, Collagenase, and Collagen Cross-Linking. Molecules. 2022; 27(22):7919. https://doi.org/10.3390/molecules27227919
Chicago/Turabian StyleLi, Huifang, Tithi Roy, Samuel T. Boateng, Hao He, Chang Liu, Weixi Liu, Dongli Li, Panpan Wu, Navindra P. Seeram, Jean Christopher Chamcheu, and et al. 2022. "Standardized Pomegranate (Pomella®) and Red Maple (Maplifa®) Extracts and Their Phenolics Protect Type I Collagen by the Inhibition of Matrix Metalloproteinases, Collagenase, and Collagen Cross-Linking" Molecules 27, no. 22: 7919. https://doi.org/10.3390/molecules27227919