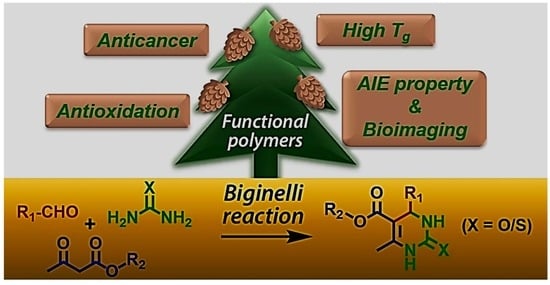
Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction
Abstract
:1. Introduction
2. Bioactive Polymers
3. Other Applications
4. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreye, O.; Toth, T.; Meier, M.A. Introducing multicomponent reactions to polymer science: Passerini reactions of renewable monomers. J. Am. Chem. Soc. 2011, 133, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.X.; Li, L.; Li, Z.L.; Lv, A.; Du, F.S.; Li, Z.C. Sequence regulated Poly(ester-amide)s based on passerini reaction. ACS Macro Lett. 2012, 1, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Solleder, S.C.; Meier, M.A.R. Sequence control in polymer chemistry through the passerini three-component reaction. Angew. Chem. Int. Ed. 2014, 53, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Sehlinger, A.; Meier, M.A.R. Passerini and Ugi multicomponent reactions in polymer science. In Multi-Component and Sequential Reactions in Polymer Synthesis; Theato, P., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2015; Volume 269, pp. 61–86. [Google Scholar]
- Zhang, J.; Zhang, M.; Du, F.S.; Li, Z.C. Synthesis of functional polycaprolactones via passerini multicomponent polymerization of 6-oxohexanoic acid and isocyanides. Macromolecules 2016, 49, 2592–2600. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.H.; Wang, J.C.; Du, F.S.; Li, Z.C. Functional Poly(ester-amide)s with tertiary ester linkages via the passerini multicomponent polymerization of a dicarboxylic acid and a diisocyanide with different electron-deficient ketones. Macromolecules 2018, 51, 5842–5851. [Google Scholar] [CrossRef]
- Oelmann, S.; Travanut, A.; Barther, D.; Romero, M.; Howdle, S.M.; Alexander, C.; Meier, M.A.R. Biocompatible unimolecular micelles obtained via the passerini reaction as versatile nanocarriers for potential medical applications. Biomacromolecules 2019, 20, 90–101. [Google Scholar] [CrossRef]
- Kreye, O.; Tueruenc, O.; Sehlinger, A.; Rackwitz, J.; Meier, M.A.R. Structurally diverse polyamides obtained from monomers derived via the Ugi multicomponent reaction. Chem. Eur. J. 2012, 18, 5767–5776. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.-Q. Ugi multicomponent reaction product: The inhibitive effect on DNA oxidation depends upon the isocyanide moiety. J. Org. Chem. 2013, 78, 8696–8704. [Google Scholar] [CrossRef]
- Sehlinger, A.; Dannecker, P.-K.; Kreye, O.; Meier, M.A.R. Diversely substituted polyamides: Macromolecular design using the Ugi four-component reaction. Macromolecules 2014, 47, 2774–2783. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Fu, C.; Zhu, C.; Zhang, Y.; Wang, S.; Wei, Y.; Tao, L. Introducing the Ugi reaction into polymer chemistry as a green click reaction to prepare middle-functional block copolymers. Polym. Chem. 2014, 5, 2704–2708. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Wei, Y.; Fu, C.; Tao, L. The Ugi reaction in polymer chemistry: Syntheses, applications and perspectives. Polym. Chem. 2015, 6, 8233–8239. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Liu, J.; Xie, Z.; Luan, S.; Xiao, C.; Tao, Y.; Wang, X. Ugi reaction of natural amino acids: A general route toward facile synthesis of polypeptoids for bioapplications. ACS Macro Lett. 2016, 5, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Dannecker, P.-K.; Sehlinger, A.; Meier, M.A.R. Polymacrocycles derived via Ugi multi-component reactions. Macromol. Rapid Commun. 2019, 40, 1800748. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, Z.; Tao, Y. Polypeptoids synthesis based on Ugi reaction: Advances and perspectives. Biopolymers 2019, 110, e23288. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Liu, G.; Wei, Y.; Wu, Y.; Tao, L. Antibacterial self-healing hydrogel via the Ugi reaction. ACS Appl. Polym. Mater. 2020, 2, 404–410. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, G.; Lv, T.; He, X.; Wei, Y.; Pan, R.; Yang, L.; Tao, L. Antioxidant polymers via the Ugi reaction for in vivo protection of UV-induced oxidative stress. Chem. Mater. 2022, 34, 2645–2654. [Google Scholar] [CrossRef]
- Wu, G.M.; Sun, W.L.; Shen, Z.Q. Synthesis and properties of two Poly(phenyl methacylate)s functionalized with pedent Dihydropyrimid(thi)one groups. Chin. J. Polym. Sci. 2009, 27, 293–296. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, B.; Zhao, Y.; Fu, C.; Tao, L.; Wei, Y. A new insight into the biginelli reaction: The dawn of multicomponent click chemistry? Polym. Chem. 2013, 4, 5395–5400. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, H.; Zhang, Y.; Wang, X.; Yang, B.; Zhang, Q.; Ren, X.; Fu, C.; Wei, Y.; Wang, Z.; et al. Postpolymerization modification of Poly(dihydropyrimidin-2(1H)-thione)s via the thiourea-haloalkane reaction to prepare functional polymers. ACS Macro Lett. 2015, 4, 843–847. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.; Zhang, Y.; Wang, X.; Yang, B.; Zhang, Y.; Zhang, Q.; Fu, C.; Wei, Y.; Tao, L. From drug to adhesive: A new application of Poly(dihydropyrimidin-2(1H)-one)s via the biginelli polycondensation. Polym. Chem. 2015, 6, 4940–4945. [Google Scholar] [CrossRef]
- Boukis, A.C.; Llevot, A.; Meier, M.A. High glass transition temperature renewable polymers via biginelli multicomponent polymerization. Macromol. Rapid Commun. 2016, 37, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhao, Y.; Wu, H.; Wang, Z.; Yang, B.; Wei, Y.; Wang, Z.; Tao, L. Multicomponent combinatorial polymerization via the biginelli reaction. J. Am. Chem. Soc. 2016, 138, 8690–8693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, H.; Wang, Z.; Wei, Y.; Wang, Z.; Tao, L. Training the old dog new tricks: The applications of the biginelli reaction in polymer chemistry. Sci. China Chem. 2016, 59, 1541–1547. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.; Tao, L. Polymer synthesis by mimicking nature’s strategy: The combination of ultra-fast RAFT and the biginelli reaction. Polym. Chem. 2017, 8, 5679–5687. [Google Scholar] [CrossRef]
- Mao, T.; Liu, G.; Wu, H.; Wei, Y.; Gou, Y.; Wang, J.; Tao, L. High throughput preparation of UV-protective polymers from essential oil extracts via the biginelli reaction. J. Am. Chem. Soc. 2018, 140, 6865–6872. [Google Scholar] [CrossRef]
- Wu, H.; Fu, C.; Zhao, Y.; Yang, B.; Wei, Y.; Wang, Z.; Tao, L. Multicomponent copolycondensates via the simultaneous hantzsch and biginelli reactions. ACS Macro Lett. 2015, 4, 1189–1193. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Q.; Li, Y.; Wang, X.; Wu, H.; Wei, Y.; Zeng, Y.; Tao, L. High-throughput preparation of antibacterial polymers from natural product derivatives via the Hantzsch reaction. iScience 2020, 23, 100754. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, Y.; Lv, T.; Mao, T.; Wei, Y.; Jia, S.; Gou, Y.; Tao, L. High-throughput preparation of radioprotective polymers via Hantzsch’s reaction for in vivo x-ray damage determination. Nat. Commun. 2020, 11, 6214. [Google Scholar] [CrossRef]
- Liu, G.; Pan, R.; Wei, Y.; Tao, L. The Hantzsch reaction in polymer chemistry: From synthetic methods to applications. Macromol. Rapid Commun. 2021, 42, e2000459. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Z.; Dai, X.; Zeng, Y.; Wei, Y.; He, X.; Yan, L.T.; Tao, L. De novo design of entropy-driven polymers resistant to bacterial attachment via multicomponent reactions. J. Am. Chem. Soc. 2021, 143, 17250–17260. [Google Scholar] [CrossRef]
- Kakuchi, R.; Theato, P. Efficient multicomponent postpolymerization modification based on Kabachnik-fields reaction. ACS Macro Lett. 2014, 3, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Yang, B.; Zhu, C.; Wei, Y.; Tao, L. ‘One pot’ synthesis of well-defined Poly(aminophosphonate)s: Time for the Kabachnik–fields reaction on the stage of polymer chemistry. Polym. Chem. 2014, 5, 1857–1862. [Google Scholar] [CrossRef]
- He, X.; Liu, G.; Tian, Y.; Mao, T.; Wu, H.; Wei, Y.; Tao, L. Antioxidant polymers via the Kabachnik-fields reaction to control cellular oxidative stress. Macromol. Biosci. 2020, 20, e1900419. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zeng, Y.; Liu, G.; Tian, Y.; Wei, Y.; Zhao, L.; Yang, L.; Tao, L. Magnetic self-healing hydrogel from difunctional polymers prepared via the Kabachnik-fields reaction. ACS Macro Lett. 2022, 11, 39–45. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, L.; Liu, G.; Wei, Y.; Zeng, Y.; Tao, L. Polymer chelator prepared via the Kabachnik–fields reaction for the in vivo prevention of heavy-metal damage. Chem. Mater. 2022, 34, 9558–9568. [Google Scholar] [CrossRef]
- Hassan, S.; Müller, T.J.J. Multicomponent syntheses based upon copper-catalyzed Alkyne-Azide cycloaddition. Adv. Synth. Catal. 2015, 357, 617–666. [Google Scholar] [CrossRef]
- Kayser, L.V.; Vollmer, M.; Welnhofer, M.; Krikcziokat, H.; Meerholz, K.; Arndtsen, B.A. Metal-free, multicomponent synthesis of pyrrole-based π-conjugated polymers from imines, acid chlorides, and alkynes. J. Am. Chem. Soc. 2016, 138, 10516–10521. [Google Scholar] [CrossRef]
- Wei, B.; Li, W.; Zhao, Z.; Qin, A.; Hu, R.; Tang, B.Z. Metal-free multicomponent tandem polymerizations of alkynes, amines, and formaldehyde toward structure- and sequence-controlled luminescent polyheterocycles. J. Am. Chem. Soc. 2017, 139, 5075–5084. [Google Scholar] [CrossRef]
- Lee, I.H.; Kim, H.; Choi, T.L. Cu-catalyzed multicomponent polymerization to synthesize a library of Poly(N-sulfonylamidines). J. Am. Chem. Soc. 2013, 135, 3760–3763. [Google Scholar] [CrossRef]
- Leitch, D.C.; Kayser, L.V.; Han, Z.Y.; Siamaki, A.R.; Keyzer, E.N.; Gefen, A.; Arndtsen, B.A. A palladium-catalysed multicomponent coupling approach to conjugated Poly(1,3-dipoles) and polyheterocycles. Nat. Commun. 2015, 6, 7411. [Google Scholar] [CrossRef]
- Kim, H.; Bang, K.T.; Choi, I.; Lee, J.K.; Choi, T.L. Diversity-oriented polymerization: One-shot synthesis of library of graft and dendronized polymers by Cu-catalyzed multicomponent polymerization. J. Am. Chem. Soc. 2016, 138, 8612–8622. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Bang, K.T.; Yang, H.S.; Choi, T.L. Recent advances in diversity-oriented polymerization using Cu-catalyzed multicomponent reactions. Macromol. Rapid Commun. 2022, 43, e2100642. [Google Scholar] [CrossRef] [PubMed]
- Blasco, E.; Sims, M.B.; Goldmann, A.S.; Sumerlin, B.S.; Barner-Kowollik, C. 50th anniversary perspective: Polymer functionalization. Macromolecules 2017, 50, 5215–5252. [Google Scholar] [CrossRef]
- Afshari, R.; Shaabani, A. Materials functionalization with multicomponent reactions: State of the art. ACS Comb. Sci. 2018, 20, 499–528. [Google Scholar] [CrossRef]
- Tian, T.; Hu, R.; Tang, B.Z. Room temperature one-step conversion from elemental sulfur to functional polythioureas through catalyst-free multicomponent polymerizations. J. Am. Chem. Soc. 2018, 140, 6156–6163. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Multicomponent reactions-based modified/functionalized materials in the biomedical platforms. ACS Appl. Bio Mater. 2020, 3, 156–174. [Google Scholar] [CrossRef] [Green Version]
- Pan, R.; Liu, G.; Zeng, Y.; He, X.; Ma, Z.; Wei, Y.; Chen, S.; Yang, L.; Tao, L. A multi-responsive self-healing hydrogel for controlled release of curcumin. Polym. Chem. 2021, 12, 2457–2463. [Google Scholar] [CrossRef]
- Zhang, J.; Zang, Q.; Yang, F.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Sulfur conversion to multifunctional Poly(O-thiocarbamate)s through multicomponent polymerizations of sulfur, diols, and diisocyanides. J. Am. Chem. Soc. 2021, 143, 3944–3950. [Google Scholar] [CrossRef]
- Peng, F.; Liu, H.; Hu, S.; Yue, F.; Xiao, D.; Guo, L.; Qi, H. High throughput preparation of antioxidant polysaccharide-based polymers with UV-resistant and antibacterial performance. Food Hydrocoll. 2022, 133, 107936. [Google Scholar] [CrossRef]
- Biginelli, P. Ueber Aldehyduramide des Acetessigäthers. Ber. Dtsch. Chem. Ges. 1891, 24, 1317–1319. [Google Scholar] [CrossRef]
- Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. Eur. J. Med. Chem. 2017, 132, 108–134. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.H.S.; Masson, F.T.; Simeoni, L.A.; Homem-de-Mello, M. Biological Activity of Dihydropyrimidinone (DHPM) derivatives: A systematic review. Eur. J. Med. Chem. 2018, 143, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yang, B.; Zhao, Y.; Zhang, X.; Wang, X.; Wei, Y.; Tao, L. One-pot polymer conjugation on carbon nanotubes through simultaneous π–π stacking and the biginelli reaction. Polymer 2015, 64, 210–215. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Y.; Li, Y.; Yang, L.; Zhao, Y.; Liu, G.; Wei, Y.; Wang, X.; Tao, L. Post-polymerization modification via the biginelli reaction to prepare water-soluble polymer adhesives. Polym. Chem. 2017, 8, 5490–5495. [Google Scholar] [CrossRef]
- Dong, J.; Liu, M.; Jiang, R.; Huang, H.; Wan, Q.; Wen, Y.; Tian, J.; Dai, Y.; Zhang, X.; Wei, Y. Synthesis and biological imaging of cross-linked fluorescent polymeric nanoparticles with aggregation-induced emission characteristics based on the combination of RAFT polymerization and the biginelli reaction. J. Colloid. Interface Sci. 2018, 528, 192–199. [Google Scholar] [CrossRef]
- Mao, T.; Yang, L.; Liu, G.; Wei, Y.; Gou, Y.; Wang, J.; Tao, L. Ferrocene-containing polymer via the biginelli reaction for in vivo treatment of oxidative stress damage. ACS Macro Lett. 2019, 8, 639–645. [Google Scholar] [CrossRef]
- Rong, L.; Zeng, M.; Liu, H.; Wang, B.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Yuan, J.; Sui, X. Biginelli reaction on cellulose acetoacetate: A new approach for versatile cellulose derivatives. Carbohydr. Polym. 2019, 209, 223–229. [Google Scholar] [CrossRef]
- Esen, E.; Meier, M.A.R. Modification of starch via the biginelli multicomponent reaction. Macromol. Rapid Commun. 2020, 41, e1900375. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tan, T.; Zhao, Y.; Wei, Y.; Wang, D.; Chen, R.; Tao, L. Anticancer polymers via the biginelli reaction. ACS Macro Lett. 2020, 9, 1249–1254. [Google Scholar] [CrossRef]
- Windbiel, J.T.; Meier, M.A.R. Synthesis of new biginelli polycondensates: Renewable materials with tunable high glass transition temperatures. Polym. Int. 2020, 70, 506–513. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, Y.; Wu, H.; Zhou, C.; Tao, L. An antioxidant self-healing hydrogel for 3D cell cultures. J. Mater. Chem. B 2020, 8, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; He, X.; Liu, G.; Wei, Y.; Gou, Y.; Zhou, X.; Tao, L. Fluorescent polymers via post-polymerization modification of biginelli-type polymers for cellular protection against UV damage. Polym. Chem. 2021, 12, 852–857. [Google Scholar] [CrossRef]
- Windbiel, J.T.; Meier, M.A.R. RAFT polymerization of a renewable ricinoleic acid-derived monomer and subsequent post-polymerization modification via the biginelli-3-component reaction. Macromol. Chem. Phys. 2021, 223, 2100360. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, C.; Tao, L. Stimuli-responsive multifunctional phenylboronic acid polymers via multicomponent reactions: From synthesis to application. Macromol. Rapid Commun. 2021, 42, e2100022. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, J.; Zhang, P.; Zhao, R.; Chen, Z.; Wang, M.; Deng, K. Polyurea modified with 4-dihydropyrimidone-2-ketone rings by biginelli reaction and its boostered AIE characteristic. Macromol. Chem. Phys. 2021, 222, 2100284. [Google Scholar] [CrossRef]
- Zhou, M.; Li, L.; Xie, W.; He, Z.; Li, J. Synthesis of a thermal-responsive dual-modal supramolecular probe for magnetic resonance imaging and fluorescence imaging. Macromol. Rapid Commun. 2021, 42, e2100248. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Zhang, X.; Lin, L. ROS-Scavenging glyco-nanoplatform for synergistic antibacterial and wound-healing therapy of bacterial keratitis. J. Mater. Chem. B 2022, 10, 4575–4587. [Google Scholar] [CrossRef]
- Sies, H. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engl. 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.X.; Sun, Y.; Deng, K.; Mei, J.Y.; Chermansky, C.J.; Damaser, M.S. Potential role of oxidative stress in the pathogenesis of diabetic bladder dysfunction. Nat. Rev. Urol. 2022, 19, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, D.D.; Cyrino, L.A.R.; Ferreira, G.K.; Magro, D.D.D.; Calegari, C.R.; Cabral, H.; Cavichioli, N.; Ramos, S.A.; Ullmann, O.M.; Mayer, Y.; et al. Neuroinflammation and neuroprogression produced by oxidative stress in euthymic bipolar patients with different onset disease times. Sci. Rep. 2022, 12, 16742. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Slatore, C.G.; Littman, A.J.; Au, D.H.; Satia, J.A.; White, E. Long-term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. Am. J. Respir. Crit. Care. Med. 2008, 177, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, A.; Xu, P.; Pindrus, M.A.; Blasioli, D.J.; Kaplan, D.L. Expansion and osteogenic differentiation of bone marrow-derived mesenchymal stem cells on a vitamin C functionalized polymer. Biomaterials 2006, 27, 3265–3273. [Google Scholar] [CrossRef] [PubMed]
- Wattamwar, P.P.; Mo, Y.; Wan, R.; Palli, R.; Zhang, Q.; Dziubla, T.D. Antioxidant activity of degradable polymer Poly(trolox ester) to suppress oxidative stress injury in the cells. Adv. Funct. Mater. 2010, 20, 147–154. [Google Scholar] [CrossRef]
- Hlushko, R.; Hlushko, H.; Sukhishvili, S.A. A family of linear phenolic polymers with controlled hydrophobicity, adsorption and antioxidant properties. Polym. Chem. 2018, 9, 506–516. [Google Scholar] [CrossRef]
- Nagarajan, S.; Nagarajan, R.; Kumar, J.; Salemme, A.; Togna, A.R.; Saso, L.; Bruno, F. Antioxidant activity of synthetic polymers of phenolic compounds. Polymers 2020, 12, 1646. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.; Hlushko, H.; Abbott, A.; Aliakseyeu, A.; Hlushko, R.; Sukhishvili, S.A. Integrating antioxidant functionality into polymer materials: Fundamentals, strategies, and applications. ACS Appl. Mater. Interfaces 2021, 13, 41372–41395. [Google Scholar] [CrossRef]
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. Recent advances in antioxidant polymers: From sustainable and natural monomers to synthesis and applications. Polymers 2021, 13, 2465. [Google Scholar] [CrossRef]
- Stefani, H.A.; Oliveira, C.B.; Almeida, R.B.; Pereira, C.M.; Braga, R.C.; Cella, R.; Borges, V.C.; Savegnago, L.; Nogueira, C.W. Dihydropyrimidin-(2H)-ones obtained by ultrasound irradiation: A new class of potential antioxidant agents. Eur. J. Med. Chem. 2006, 41, 513–518. [Google Scholar] [CrossRef]
- Jager, T.L.d.; Cockrell, A.E.; Plessis, S.S.D. Ultraviolet light induced generation of reactive oxygen species. In Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S.I., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 996, pp. 15–23. [Google Scholar]
- Liu, Z.Q. Enhancing antioxidant effect against peroxyl radical-induced oxidation of DNA: Linking with ferrocene moiety! Chem. Rec. 2019, 19, 2385–2397. [Google Scholar] [CrossRef]
- Liu, Z.Q. Multicomponent reactions for integrating multiple functional groups into an antioxidant. Chem. Rec. 2020, 20, 1516–1529. [Google Scholar] [CrossRef]
- Staveren, D.R.v.; Metzler-Nolte, N. Bioorganometallic chemistry of ferrocene. Chem. Rev. 2004, 104, 5931–5985. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.-Q. Ferrocene as a functional group enhances the inhibitive effect of dihydropyrimidine on radical-induced oxidation of DNA. Org. Chem. Front. 2014, 1, 792–797. [Google Scholar] [CrossRef]
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial kratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Hilliam, Y.; Kaye, S.; Winstanley, C. Pseudomonas aeruginosa and microbial keratitis. J. Med. Microbiol. 2020, 69, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef]
- Forbes, S.J.; Rosenthal, N. Preparing the ground for tissue regeneration: From mechanism to therapy. Nat. Med. 2014, 20, 857–869. [Google Scholar] [CrossRef]
- Mayer, T.U.; Kapoor, T.M.; Haggarty, S.J.; King, R.W.; Schreiber, S.L.; Mitchison, T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 1999, 186, 971–974. [Google Scholar] [CrossRef] [Green Version]
- Guido, B.C.; Ramos, L.M.; Nolasco, D.O.; Nobrega, C.C.; Andrade, B.Y.; Pic-Taylor, A.; Neto, B.A.; Correa, J.R. Impact of kinesin Eg5 Inhibition by 3,4-Dihydropyrimidin-2(1H)-one derivatives on various breast cancer cell features. BMC Cancer 2015, 15, 283. [Google Scholar] [CrossRef] [Green Version]
- Bhat, M.A.; Al-Dhfyan, A.; Al-Omar, M.A. Targeting cancer stem cells with novel 4-(4-Substituted phenyl)-5-(3,4,5-trimethoxy/3,4-dimethoxy)-benzoyl-3,4-dihydropyrimidine-2(1H)-one/thiones. Molecules 2016, 21, 1746. [Google Scholar] [CrossRef] [Green Version]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Gondru, R.; Peddi, S.R.; Manga, V.; Khanapur, M.; Gali, R.; Sirassu, N.; Bavantula, R. One-pot synthesis, biological evaluation and molecular docking studies of fused Thiazolo[2,3-b]pyrimidinone-pyrazolylcoumarin hybrids. Mol. Divers. 2018, 22, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zeng, Y.; He, X.; Pan, S.; Wei, Y.; Wang, B.; Tao, L. Introducing the aza-michael addition reaction between acrylate and Dihydropyrimidin-2(1H)-thione into polymer chemistry. Polym. Chem. 2022. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Wang, B.; Tao, L. Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction. Molecules 2022, 27, 7886. https://doi.org/10.3390/molecules27227886
Ma Z, Wang B, Tao L. Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction. Molecules. 2022; 27(22):7886. https://doi.org/10.3390/molecules27227886
Chicago/Turabian StyleMa, Zeyu, Bo Wang, and Lei Tao. 2022. "Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction" Molecules 27, no. 22: 7886. https://doi.org/10.3390/molecules27227886