Integrating Multi-Type Component Determination and Anti-Oxidant/-Inflammatory Assay to Evaluate the Impact of Pre-Molting Washing on the Quality and Bioactivity of Cicadae Periostracum
Abstract
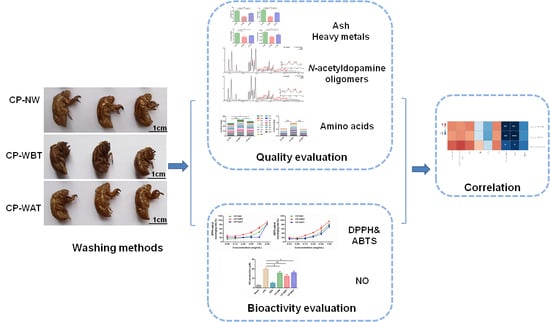
:1. Introduction
2. Results and Discussion
2.1. Comparison of Total Ash and Acid-Insoluble Ash in Three Kinds of CP Samples
2.2. Comparison of Four Heavy Metals in Three Kinds of CP Samples
2.3. Qualitative and Quantitative Comparison of N-acetyldopamine Oligomers in Three Kinds of CP Samples
2.4. Qualitative and Quantitative Comparisons of Amino Acids in Three Kinds of CP Samples
2.5. Comparison of DPPH and ABTS Radical Scavenging Rates of Three Kinds of CP Samples
2.6. Comparison of Nitric Oxide (NO) Production among Three Kinds of CP Samples
2.7. Correlations between Contents of Multi-Type Components and Anti-Oxidant/-Inflammatory Activities
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Sample Collection and Primary Processing
3.3. Determination of Total Ash and Acid-Insoluble Ash
3.4. Determination of Common Heavy Metals
3.5. Determination of N-acetyldopamine Oligomers
3.6. Determination of Amino Acids
3.7. Determination of DPPH and ABTS Radical Scavenging Rate
3.8. Determination of Nitric Oxide (NO)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Publisher: Beijing, China, 2020; p. 385. [Google Scholar]
- Zhang, Q.; Li, R.L.; Tao, T.; Sun, J.Y.; Liu, J.; Zhang, T.; Peng, W.; Wu, C.J. Antiepileptic effects of cicadae periostracum on mice and its antiapoptotic effects in H2O2-stimulated PC12 cells via regulation of PI3K/Akt/Nrf2 signaling pathways. Oxid. Med. Cell. Longev. 2021, 2021, 5598818. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Ye, K.; Jiang, Q.; Wang, M.; Wen, X.; Yang, J. Chemical profile of Xian-He-Cao-Chang-Yan formula and its effects on ulcerative colitis. J. Ethnopharmacol. 2021, 267, 113517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, W.D.; Cao, L. Determination of Peimine and Peiminine in Huangshi Xinagsheng Pills by HPLC-ELSD. Drug Stand. China 2010, 11, 369–372. [Google Scholar]
- Ye, M.; Luo, G.; Ye, D.; Hong, G.; Hong, W.S.; Ju, W.Z. Network pharmacology, molecular docking integrated surface plasmon resonance technology reveals the mechanism of Toujie Quwen Granules against coronavirus disease 2019 pneumonia. Phytomedicine 2021, 85, 153401. [Google Scholar] [CrossRef]
- Zhao, R.H.; Han, J.; Han, Z.L.; Zhang, S.H.; Lin, J.T. Meta-analysis of efficacy and safety of Suhuang Zhike Capsules in treatment of chronic obstructive pulmonary disease. Chin. J. Chin. Mater. Med. 2022, 47, 1095–1102. [Google Scholar]
- Chou, I.; Lei, Z.R.; Li, L.; Lu, X.L.; Yao, W. The Cicadidae of China; Tianze Publishing House: Hong Kong, China, 1997; p. 24. [Google Scholar]
- Xu, M.Z.; Lee, W.S.; Han, J.M.; Oh, H.W.; Park, D.S.; Tian, G.R.; Jeong, T.S.; Park, H.Y. Antioxidant and anti-inflammatory activities of N-acetyldopamine dimers from Periostracum Cicadae. Bioorg. Med. Chem. 2006, 14, 7826–7834. [Google Scholar] [CrossRef]
- Yen, H.R.; Liang, K.L.; Huang, T.P.; Fan, J.Y.; Chang, T.T.; Sun, M.F. Characteristics of traditional Chinese medicine use for children with allergic rhinitis: A nationwide population-based study. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 591–597. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.H.; Yang, W.K.; Kim, H.J.; An, H.J.; Lee, Y.C. Cryptotympana pustulata extract and its main active component, oleic acid, inhibit ovalbumin-induced allergic airway inflammation through inhibition of Th2/GATA-3 and interleukin-17/RORγt signaling pathways in asthmatic mice. Molecules 2021, 26, 1854. [Google Scholar] [CrossRef]
- Cao, X.C.; Zhang, X.Y.; Xu, J.D.; Shen, H.; Zhou, S.S.; Zhu, H.; Kong, M.; Zhang, W.; He, Y.; Mao, Q.; et al. Quality consistency evaluation on four origins of Cicadae Periostracum by ultra-performance liquid chromatography coupled with quadrupole/time-of-flight mass spectrometry analysis. J. Pharm. Biomed. Anal. 2020, 179, 112974. [Google Scholar] [CrossRef]
- Cao, X.C.; Guo, M.F.; Han, Y.; Fan, Y.T.; Zhu, J.H.; Zhu, H.; Xu, J.D.; Shen, H.; Zhou, G.R.; Mao, Q.; et al. Systematic metabolite profiling of N-acetyldopamine oligomers from Cicadae Periostracum in rats by ultra-high performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2021, 192, 113665. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, J.S.; Wang, G.K.; Zheng, J.; Liu, S.Z. Determination of three amino acids in cicada slough by pre-column derivatization reversed-phase high-performance liquid chromatography. J. Anhui Univ. Chin. Med. 2017, 36, 369–372. [Google Scholar]
- Committee of Traditional Chinese Medicine, Department of Health. Taiwan Herbal Pharmacopoeia; Version III; Executive Yuan Publisher: Taiwan, China, 2018; p. 435. [Google Scholar]
- Zuo, T.T.; Li, Y.L.; He, H.Z.; Jin, H.Y.; Zhang, L.; Sun, L.; Gao, F.; Wang, Q.; Shen, Y.J.; Ma, S.C. Refined assessment of heavy metal-associated health risk due to the consumption of traditional animal medicines in humans. Environ. Monit. Assess. 2019, 191, 171. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yang, X.Y.; Xiao, J.J.; Liang, S.X.; Lin, Z.S.; Li, J.; Zhang, X.G.; Lv, G.H. Determination of heavy metal elements in Chuanmingshen violaceum by ICP-MS. Chin. Tradit. Herb. Drugs 2016, 47, 1595–1600. [Google Scholar]
- Chen, Y.G.; He, X.; Huang, J.H.; Luo, R.; Ge, H.Z.; Wolowicz, A.; Wawrzkiewicz, M.; Płaska, A.G.; Li, B.; Yu, Q.X.; et al. Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotoxicol. Environ. Saf. 2021, 219, 112336. [Google Scholar] [CrossRef]
- Cooper, K.; Noller, B.; Connell, D.; Yu, J.; Sadler, R.; Olszowy, H.; Golding, G.; Tinggi, U.; Moore, M.R.; Myers, S. Public health risks from heavy metals and metalloids present in traditional Chinese medicines. J. Toxicol. Environ. Health A 2007, 70, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.T.; Qu, H.R.; Jin, H.Y.; Zhang, L.; Luo, F.Y.; Yu, K.Z.; Gao, F.; Wang, Q.; Sun, L.; He, H.Z.; et al. Innovative health risk assessments of heavy metals based on bioaccessibility due to the consumption of traditional animal medicines. Environ. Sci. Pollut. R. 2020, 27, 22593–22603. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.G.; Wang, H.Y.; Zhang, K.; Zhu, Y.; Zhao, W.B.; Wang, H.; Wang, J.H. Five new N-acetyldopamine dimers from Periostracum Cicadae. Phytochem. Lett. 2016, 16, 97–102. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Y.M.; Liao, L.; Wang, S.X.; Zhang, Y.; Cheng, Y.X. Cicadamides A and B, N-acetyldopamine dimers from the insect Periostracum Cicadae. Nat. Prod. Commun. 2019, 6, 1–6. [Google Scholar]
- Yang, L.; Li, G.Y.; Li, Q.R.; Wang, J.H. Two new N-acetyldopamine tetrapolymers from periostracum Cicadae. J. Asian Nat. Prod. Res. 2012, 3, 204–209. [Google Scholar] [CrossRef]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef]
- Matsumoto, H.; Konno, K.; Kazumaa, K. A comprehensive LC-MS and isolation study of cicada slough as a crude drug. Nat. Prod. Commun. 2017, 12, 1789–1792. [Google Scholar] [CrossRef] [Green Version]
- Olin-Sandoval, V.; Yu, J.S.L.; Miller-Fleming, L.; Alam, M.T.; Kamrad, S.; Correia-Melo, C.; Haas, R.; Segal, J.; Pena Navarro, D.A.; Herrera-Dominguez, L.; et al. Lysine harvesting is an antioxidant strategy and triggers underground polyamine metabolism. Nature 2019, 572, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.C.; Wang, Z.X.; Li, H.; Cai, L.; Pan, J.H.; He, H.J.; Wu, Q.; Tang, Y.Z.; Ma, J.P.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, X.R.; Li, J.; Zang, Y.; Li, X. Selective and sensitive fluorescence imaging reveals microenvironment-dependent behavior of NO modulators in the endothelial system. J. Pharm. Anal. 2020, 10, 466–472. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, C.; Yu, Y.B.; Wu, L.X.; Chen, T.T.; Wang, Z.W. Physicochemical characteristics and in vitro biological activities of polysaccharides derived from raw garlic (Allium sativum L.) bulbs via three-phase partitioning combined with gradient ethanol precipitation method. Food Chem. 2021, 339, 128081. [Google Scholar] [CrossRef]
- De Angelo, J. Nitric oxide scavengers in the treatment of shock associated with systemic inflflammatory response syndrome. Expert Opin. Pharmacother. 1999, 1, 19–29. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khaket, T.P.; Bajpai, V.K.; Shukla, S.; Park., I.; Na, M.; Huh, Y.S.; Han, Y.K.; Kang, S.C.; Kim, M. N-Acetyldopamine dimers from Oxya chinensis sinuosa attenuates lipopolysaccharides induced inflammation and inhibits cathepsin C activity. Comput. Struct. Biotechnol. J. 2022, 20, 1177–1188. [Google Scholar] [CrossRef]
- Jungnickel, K.E.J.; Parker, J.L.; Newstead, S. Structural basis for amino acid transport by the CAT family of SLC7 transporters. Nat. Commun. 2018, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Song, J.J.; Li, T.G.; Cheng, X.; Ji, X.M.; Gao, D.X.; Du, M.; Jiang, N.Y.; Liu, X.L.; Mao, X.Y. Sea cucumber peptides exert anti-inflammatory activity through suppressing NF-κB and MAPK and inducing HO-1 in RAW264.7 macrophages. Food Funct. 2016, 7, 2773–2779. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Uslu, N.; Musa Ozcan, M.; Al Juhaimi, F.; Ghafoor, K.; Babiker, E.E.; Osman, M.A.; Alqah, H.A.S. Effect of conventional oven roasting treatment on the physicochemical quality attributes of sesame seeds obtained from different locations. Food Chem. 2021, 338, 128109. [Google Scholar] [CrossRef]
- Kukusamude, C.; Sricharoen, P.; Limchoowong, N.; Kongsri, S. Heavy metals and probabilistic risk assessment via rice consumption in Thailand. Food Chem. 2021, 334, 127402. [Google Scholar] [CrossRef] [PubMed]
- Thabit, T.; Elgeddawy, D.I.H.; Shokr, S.A. Determination of some common heavy metals and radionuclides in some medicinal herbs using ICP-MS/MS. J. AOAC. Int. 2020, 103, 1282–1287. [Google Scholar] [CrossRef]
- Wan, J.Y.; Fan, Y.; Yu, Q.T.; Ge, Y.Z.; Yan, C.P.; Alolga, R.N.; Li, P.; Ma, Z.H.; Qi, L.W. Integrated evaluation of malonyl ginsenosides, amino acids and polysaccharides in fresh and processed ginseng. J. Pharm. Biomed. Anal. 2015, 107, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, D.; Liu, H.; Wang, Z.; Hui, T.; Sun, J. Comprehensive evaluation of volatile and nonvolatile compounds in oyster cuts of roasted lamb at different processing stages using traditional nang roasting. Foods 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yu, X.; Fang, J.; Lu, Y.; Liu, P.; Xing, Y.; Wang, Q.; Che, Z.; He, Q. Flavor compounds in Pixian broad-bean paste: Non-volatile organic acids and amino acids. Molecules 2018, 23, 1299. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, T.; Choi, Y.W.; Kim, Y.K. Effect of an extraction solvent on the antioxidant quality of Pinus densiflora needle extract. J. Pharm. Anal. 2019, 9, 193–200. [Google Scholar] [CrossRef]
- Sakemi, Y.; Hagiwara, M.; Oikawa, A.; Sato, M.; Sato, S.; Sawa, N.; Nishizawa, H.; Shindo, K. Antioxidant p-terphenyl compounds in the mushroom Boletopsis leucomelas (PERS.) FAYOD and how they change via cooking. Food Chem. 2021, 363, 130281. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, S.; Huang, Q.; Tan, J.; Zeng, J.; Yao, J.; Feng, T.; Wang, G.; Zhang, Y. The lipid lowering and antioxidative stress potential of polysaccharide from Auricularia auricula prepared by enzymatic method. Int. J. Biol. Macromol. 2021, 187, 651–663. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Wen, H.; Jiang, M.; Wu, F.; Tian, J. Synthesis and evaluation of acetylferulic paeonol ester and ferulic paeonol ester as potential antioxidants to inhibit fish oil oxidation. Food Chem. 2021, 365, 130384. [Google Scholar] [CrossRef]
- Wu, C.N.; Sun, L.C.; Chu, Y.L.; Yu, R.C.; Hsieh, C.W.; Hsu, H.Y.; Hsu, F.C.; Cheng, K.C. Bioactive compounds with anti-oxidative and anti-inflammatory activities of hop extracts. Food Chem. 2020, 330, 127244. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Chen, L.; Liu, R.; Chang, M.; Wang, X. Identification and in vitro anti-inflammatory activity of different forms of phenolic compounds in Camellia oleifera oil. Food Chem. 2021, 344, 128660. [Google Scholar] [CrossRef] [PubMed]





| No. | tR (min) | Formula | Compound | Molecular Ion | Calcd. (m/z) | Exptl. (m/z) | Error (ppm) | Fragment Ion | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.79 | C20H22N2O6 | N-acetyldopamine dimer A * | [M+H]+ | 387.1556 | 387.1549 | −1.8 | 328.1179, | [11,12,20,21] |
| 269.0809, | |||||||||
| 192.0653, | |||||||||
| 150.0550 | |||||||||
| 2 | 2.85 | C20H22N2O6 | N-acetyldopamine dimers | [M+H]+ | 387.1556 | 387.1550 | −1.5 | 328.1178, | [11,12,20,21] |
| 269.0808, | |||||||||
| 192.0654, | |||||||||
| 150.0548 | |||||||||
| 3 | 3.01 | C20H22N2O6 | N-acetyldopamine dimer B * | [M+H]+ | 387.1556 | 387.1550 | −1.5 | 328.1180, | [11,12,20,21] |
| 269.0805, | |||||||||
| 192.0653, | |||||||||
| 150.0545 | |||||||||
| 4 | 3.36 | C20H22N2O6 | N-acetyldopamine dimers | [M+H]+ | 387.1556 | 387.1551 | −1.3 | 328.1178, | [11,12,20,21] |
| 269.0807, | |||||||||
| 192.0656, | |||||||||
| 150.0551 | |||||||||
| 5 | 3.60 | C20H20N2O6 | N-acetyldopamine dimers side-chain isomer | [M+H]+ | 385.1400 | 385.1394 | −1.6 | 326.1022, | [11,12] |
| 192.0658, | |||||||||
| 150.0556 | |||||||||
| 6 | 4.24 | C30H31N3O9 | N-acetyldopamine trimers | [M+H]+ | 578.2139 | 578.2141 | 0.3 | 387.1544, | [11,12] |
| 385.1392, | |||||||||
| 192.0654 | |||||||||
| 7 | 4.34 | C30H31N3O9 | N-acetyldopamine trimers | [M+H]+ | 578.2139 | 578.2137 | −0.3 | 387.1543, | [11,12] |
| 385.1392, | |||||||||
| 192.0653 | |||||||||
| 8 | 4.44 | C30H31N3O9 | N-acetyldopamine trimers | [M+H]+ | 578.2139 | 578.2139 | 0.0 | 387.1543, | [11,12] |
| 385.1390, | |||||||||
| 192.0654 | |||||||||
| 9 | 4.52 | C30H31N3O9 | N-acetyldopamine trimers | [M+H]+ | 578.2139 | 578.2137 | −0.3 | 387.1549, | [11,12] |
| 385.1390, | |||||||||
| 192.0654 | |||||||||
| 10 | 4.64 | C30H31N3O9 | N-acetyldopamine trimers | [M+H]+ | 578.2139 | 578.2140 | 0.2 | 387.1547, | [11,12] |
| 385.1396, | |||||||||
| 192.0656 | |||||||||
| 11 | 4.75 | C30H31N3O9 | N-acetyldopamine trimers | [M+H]+ | 578.2139 | 578.2144 | 0.9 | 387.1550, | [11,12] |
| 385.1385, | |||||||||
| 192.0656 | |||||||||
| 12 | 4.92 | C30H31N3O9 | N-acetyldopamine trimers | [M+H]+ | 578.2139 | 578.2139 | 0.0 | 387.1544, | [11,12] |
| 385.1389, | |||||||||
| 192.0655 | |||||||||
| 13 | 5.01 | C30H29N3O9 | N-acetyldopamine trimers side-chain isomer | [M+H]+ | 576.1982 | 576.1982 | 0.0 | 517.1608, | [11,12] |
| 192.0656 | |||||||||
| 14 | 5.12 | C30H31N3O9 | N-acetyldopamine trimers | [M+H]+ | 578.2139 | 578.2137 | −0.3 | 387.1537, | [11,12] |
| 192.0656 | |||||||||
| 15 | 5.20 | C30H29N3O9 | N-acetyldopamine trimers side-chain isomer | [M+H]+ | 576.1982 | 576.1980 | −0.3 | 517.1620, | [11,12] |
| 192.0655 | |||||||||
| 16 | 5.28 | C40H40N4O12 | N-acetyldopamine tetramers | [M+H]+ | 769.2721 | 769.2724 | 0.4 | 576.1981, | [11,12] |
| 192.0657 | |||||||||
| 17 | 5.34 | C40H40N4O12 | N-acetyldopamine tetramers | [M+H]+ | 769.2721 | 769.2725 | 0.5 | 576.1978, | [11,12,22] |
| 192.0657 | |||||||||
| 18 | 5.44 | C40H40N4O12 | N-acetyldopamine tetramers | [M+H]+ | 769.2721 | 769.2720 | −0.1 | 576.1976, | [11,12,22] |
| 192.0654 | |||||||||
| 19 | 5.49 | C40H40N4O12 | N-acetyldopamine tetramers | [M+H]+ | 769.2721 | 769.2719 | −0.3 | 576.1972, | [11,12,22] |
| 192.0653 | |||||||||
| 20 | 5.56 | C40H40N4O12 | N-acetyldopamine tetramers | [M+H]+ | 769.2721 | 769.2719 | −0.3 | 576.1973, | [11,12,22] |
| 192.0652 | |||||||||
| 21 | 5.63 | C40H40N4O12 | N-acetyldopamine tetramers | [M+H]+ | 769.2721 | 769.2715 | −0.8 | 576.1976, | [11,12,22] |
| 192.0656 | |||||||||
| 22 | 5.73 | C40H40N4O12 | N-acetyldopamine tetramers | [M+H]+ | 769.2721 | 769.2725 | 0.5 | 576.1978, | [11,12,22] |
| 192.0655 | |||||||||
| 23 | 5.80 | C40H40N4O12 | N-acetyldopamine tetramers | [M+H]+ | 769.2721 | 769.2720 | −0.1 | 576.1972, | [11,12,22] |
| 192.0654 | |||||||||
| 24 | 5.91 | C40H40N4O12 | N-acetyldopamine tetramers | [M+H]+ | 769.2721 | 769.2720 | −0.1 | 576.1978, | [11,12,22] |
| 192.0655 | |||||||||
| 25 | 6.00 | C40H40N4O12 | N-acetyldopamine tetramers | [M+H]+ | 769.2721 | 769.2726 | 0.6 | 576.1980, | [11,12,22] |
| 192.0657 | |||||||||
| 26 | 6.14 | C50H49N5O15 | N-acetyldopamine pentamers | [M+H]+ | 960.3303 | 960.3306 | 0.3 | 767.2559, | [11,12] |
| 576.1974, | |||||||||
| 192.0654 | |||||||||
| 27 | 6.20. | C50H49N5O15 | N-acetyldopamine pentamers | [M+H]+ | 960.3303 | 960.3310 | 0.7 | 767.2560, | [11,12] |
| 576.1970, | |||||||||
| 192.0654 | |||||||||
| 28 | 6.35 | C50H49N5O15 | N-acetyldopamine pentamers | [M+H]+ | 960.3303 | 960.3308 | 0.5 | 767.2560, | [11,12] |
| 576.1967, | |||||||||
| 192.0654 | |||||||||
| 29 | 6.41 | C50H49N5O15 | N-acetyldopamine pentamers | [M+H]+ | 960.3303 | 960.3315 | 1.2 | 767.2560, | [11,12] |
| 576.1974, | |||||||||
| 192.0654 | |||||||||
| 30 | 6.45 | C50H49N5O15 | N-acetyldopamine pentamers | [M+H]+ | 960.3303 | 960.3317 | 1.5 | 767.2570, | [11,12] |
| 576.1967, | |||||||||
| 192.0655 | |||||||||
| 31 | 6.63 | C50H49N5O15 | N-acetyldopamine pentamers | [M+H]+ | 960.3303 | 960.3312 | 0.9 | 767.2561, | [11,12] |
| 576.1967, | |||||||||
| 192.0656 | |||||||||
| 32 | 6.68 | C50H49N5O15 | N-acetyldopamine pentamers | [M+H]+ | 960.3303 | 960.3316 | 1.4 | 767.2559, | [11,12] |
| 576.1979, | |||||||||
| 192.0658 | |||||||||
| 33 | 6.85 | C50H49N5O15 | N-acetyldopamine pentamers | [M+H]+ | 960.3303 | 960.3303 | 0.0 | 767.2558, | [11,12] |
| 576.1978, | |||||||||
| 192.0656 | |||||||||
| 34 | 6.91 | C50H49N5O15 | N-acetyldopamine pentamers | [M+H]+ | 960.3303 | 960.3309 | 0.6 | 767.2559, | [11,12] |
| 576.1981, | |||||||||
| 192.0652 | |||||||||
| 35 | 6.99 | C30H29N3O9 | N-acetyldopamine trimers side-chain isomer | [M+H]+ | 576.1982 | 576.1981 | −0.2 | 517.1597, | - |
| 192.0656 | |||||||||
| 36 | 7.18 | C30H29N3O9 | N-acetyldopamine trimers side-chain isomer | [M+H]+ | 576.1982 | 576.1979 | −0.5 | 517.1615,192.0656 | - |
| 37 | 7.36 | C30H29N3O9 | N-acetyldopamine trimers side-chain isomer | [M+H]+ | 576.1982 | 576.1978 | −0.7 | 192.0656 | - |
| 38 | 7.40 | C30H29N3O9 | N-acetyldopamine trimers side-chain isomer | [M+H]+ | 576.1982 | 576.1983 | 0.2 | 192.0654 | - |
| 39 | 7.58 | C30H29N3O9 | N-acetyldopamine trimers side-chain isomer | [M+H]+ | 576.1982 | 576.1976 | −1.0 | 192.0656 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.-F.; Zhang, H.-H.; Zhong, P.; Xu, J.-D.; Zhou, S.-S.; Long, F.; Kong, M.; Mao, Q.; Li, S.-L. Integrating Multi-Type Component Determination and Anti-Oxidant/-Inflammatory Assay to Evaluate the Impact of Pre-Molting Washing on the Quality and Bioactivity of Cicadae Periostracum. Molecules 2022, 27, 7683. https://doi.org/10.3390/molecules27227683
Guo M-F, Zhang H-H, Zhong P, Xu J-D, Zhou S-S, Long F, Kong M, Mao Q, Li S-L. Integrating Multi-Type Component Determination and Anti-Oxidant/-Inflammatory Assay to Evaluate the Impact of Pre-Molting Washing on the Quality and Bioactivity of Cicadae Periostracum. Molecules. 2022; 27(22):7683. https://doi.org/10.3390/molecules27227683
Chicago/Turabian StyleGuo, Meng-Fei, Huan-Huan Zhang, Ping Zhong, Jin-Di Xu, Shan-Shan Zhou, Fang Long, Ming Kong, Qian Mao, and Song-Lin Li. 2022. "Integrating Multi-Type Component Determination and Anti-Oxidant/-Inflammatory Assay to Evaluate the Impact of Pre-Molting Washing on the Quality and Bioactivity of Cicadae Periostracum" Molecules 27, no. 22: 7683. https://doi.org/10.3390/molecules27227683