Molecular Hybrids of Pyazolo[3,4-b]pyridine and Triazole: Design, Synthesis and In Vitro Antibacterial Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design and Evaluation of Physico-Chemical Properties
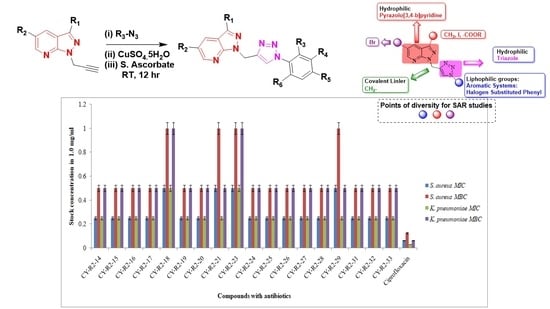
2.2. Chemistry
2.3. In Vitro Anti-Bacterial Studies
2.4. Materials and Methods
2.4.1. Chemistry
2.4.2. In Vitro Assay for Evaluation of Antibacterial Activity
Qualitative Test (Agar Well Diffusion Method)
Quantitative Estimation (Minimum Inhibitory Concentration)
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Septimus, E.J. Antimicrobial Resistance: An Antimicrobial/Diagnostic Stewardship and Infection Prevention Approach. Med. Clin. N. Am. 2018, 102, 819–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan American Health Organization. 2021 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. 2022. Available online: https://www.paho.org/en/documents/2021-antibacterial-agents-clinical-and-preclinical-development-overview-and-analysis (accessed on 20 June 2022).
- Maltezou, H.C.; Kontopidou, F.; Katerelos, P.; Daikos, G.; Roilides, E.; Theodoridou, M. Infections Caused by Carbapenem-resistant Gram-negative Pathogens in Hospitalized Children. Pediatr. Infect. Dis. J. 2013, 32, e151–e154. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Viale, P.; Viscoli, C.; Trecarichi, E.M.; Tumietto, F.; Marchese, A.; Spanu, T.; Ambretti, S.; Ginocchio, F.; Cristini, F.; et al. Predictors of Mortality in Bloodstream Infections Caused by Klebsiella pneumoniae Carbapenemase–Producing K. pneumoniae: Importance of Combination Therapy. Clin. Infect. Dis. 2012, 55, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Hobson Claire, A.; Pierrat, G.; Tenaillon, O.; Bonacorsi, S.; Bercot, B.; Jaouen, E.; Jacquier, H.; Birgy, A. Klebsiella pneumoniae Carbapenemase Variants Resistant to Ceftazidime-Avibactam: An Evolutionary Overview. Antimicrob. Agents Chemother. 2022, 66, e00447-22. [Google Scholar] [CrossRef]
- Tabah, A.; Laupland, K.B. Update on Staphylococcus aureus bacteraemia. Curr. Opin. Crit. Care 2022, 28, 495–504. [Google Scholar] [CrossRef]
- Nourollahpour Shiadeh, M.; Sepidarkish, M.; Mollalo, A.; As’adi, N.; Khani, S.; Shahhosseini, Z.; Danesh, M.; Esfandyari, S.; Mokdad, A.H.; Rostami, A. Worldwide prevalence of maternal methicillin-resistant Staphylococcus aureus colonization: A systematic review and meta-analysis. Microb. Pathog. 2022, 171, 105743. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Donaire-Arias, A.; Montagut, A.M.; Puig de la Bellacasa, R.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. 1H-Pyazolo[3,4-b]pyridines: Synthesis and Biomedical Applications. Molecules 2022, 27, 2237. [Google Scholar] [CrossRef]
- Wenglowsky, S. Pyazolo[3,4-b]pyridine kinase inhibitors: A patent review (2008–present). Expert Opin. Ther. Pat. 2013, 23, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Saxena, M.; Rishi, N. An Overview of Recent Advances in Biomedical Applications of Click Chemistry. Bioconjug. Chem. 2021, 32, 1455–1471. [Google Scholar] [CrossRef]
- Xu, Z. 1,2,3-Triazole-containing hybrids with potential antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 206, 112686. [Google Scholar] [CrossRef]
- Tan, Z.; Deng, J.; Ye, Q.; Zhang, Z. Triazole-containing hybrids with anti-Mycobacterium tuberculosis potential—Part I: 1,2,3-Triazole. Future Med. Chem. 2021, 13, 643–662. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J. The Antibacterial Activity of 1,2,3-triazole- and 1,2,4-Triazole-containing Hybrids against Staphylococcus aureus: An Updated Review (2020- Present). Curr. Top. Med. Chem. 2022, 22, 41–63. [Google Scholar] [CrossRef]
- Upadhyay, C.H. Coumarin-1,2,3-triazole Hybrid Molecules: An Emerging Scaffold for Combating Drug Resistance. Curr. Top. Med. Chem. 2021, 21, 737–752. [Google Scholar] [CrossRef]
- Hublikar, M.; Kadu, V.; Dublad, J.K.; Raut, D.; Shirame, S.; Makam, P.; Bhosale, R. (E)-2-(2-Allylidenehydrazinyl) thiazole derivatives: Design, green synthesis, in silico and in vitro antimycobacterial and radical scavenging studies. Arch. Pharm. 2020, 353, 2000003. [Google Scholar] [CrossRef]
- Parameshwar, M.; Ramkishore, M. “Big Three” Infectious Diseases: Tuberculosis, Malaria and HIV/AIDS. Curr. Top. Med. Chem. 2021, 21, 2779–2799. [Google Scholar]
- Anaikutti, P.; Selvaraj, M.; Prabhakaran, J.; Pooventhiran, T.; Jeyakumar, T.C.; Thomas, R.; Makam, P. Indolyl-4H-chromenes: Multicomponent one-pot green synthesis, in vitro and in silico, anticancer and antioxidant studies. J. Mol. Struct. 2022, 1266, 133464. [Google Scholar] [CrossRef]
- Hublikar, M.; Kadu, V.; Raut, D.; Shirame, S.; Anbarasu, S.; Al-Muhanna, M.K.; Makam, P.; Bhosale, R. 3-Substituted-2-oxindole derivatives: Design, synthesis and their anti-tuberculosis and radical scavenging dual-action studies. J. Mol. Struct. 2022, 1261, 132903. [Google Scholar] [CrossRef]
- Matsa, R.; Makam, P.; Sethi, G.; Thottasseri, A.A.; Kizhakkandiyil, A.R.; Ramadas, K.; Mariappan, V.; Pillai, A.B.; Kannan, T. Pyridine appended 2-hydrazinylthiazole derivatives: Design, synthesis, in vitro and in silico antimycobacterial studies. RSC Adv. 2022, 12, 18333–18346. [Google Scholar] [CrossRef]
- Huuskonen, J.; Livingstone, D.J.; Manallack, D.T. Prediction of drug solubility from molecular structure using a drug-like training set. SAR QSAR Environ. Res. 2008, 19, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Glynn, S.L.; Wright, J.L.; Hawi, A. Correlating Partitioning and Caco-2 Cell Permeability of Structurally Diverse Small Molecular Weight Compounds. Pharm. Res. 1998, 15, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Ungell, A.-L.; Löfroth, J.-E. Selective Paracellular Permeability in Two Models of Intestinal Absorption: Cultured Monolayers of Human Intestinal Epithelial Cells and Rat Intestinal Segments. Pharm. Res. 1993, 10, 1123–1129. [Google Scholar] [CrossRef]
- Sharifi, M.; Ghafourian, T. Estimation of Biliary Excretion of Foreign Compounds Using Properties of Molecular Structure. AAPS J. 2014, 16, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Varma, M.V.; Lai, Y.; El-Kattan, A.F. Molecular properties associated with transporter-mediated drug disposition. Adv. Drug Deliv. Rev. 2017, 116, 92–99. [Google Scholar] [CrossRef]
- Struck, S.; Schmidt, U.; Gruening, B.; Jaeger, I.S.; Hossbach, J.; Preissner, R. Toxicity versus potency: Elucidation of toxicity properties discriminating between toxins, drugs, and natural compounds. In Genome Informatics 2008; Imperial College Press: London, UK, 2008; pp. 231–242. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Leeson, P.D.; Davis, A.M. Time-related differences in the physical property profiles of oral drugs. J. Med. Chem. 2004, 47, 6338–6348. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 2011, 10, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, S.; Wong, H.; Hop, C.C.A. ADME properties and their dependence on physicochemical properties. In Drug Metabolism and Pharmacokinetics Quick Guide; Springer: New York, NY, USA, 2011; pp. 165–181. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Gaywood, A.P.; McNab, H. 3-Hydroxypyrrolo [2,3-b]pyridine and related compounds—Indoxyl analogues with fused electron deficient rings. Org. Biomol. Chem. 2010, 8, 5166–5173. [Google Scholar] [CrossRef]
- Moir, M.; Lane, S.; Lai, F.; Connor, M.; Hibbs, D.E.; Kassiou, M. Strategies to develop selective CB2 receptor agonists from indole carboxamide synthetic cannabinoids. Eur. J. Med. Chem. 2019, 180, 291–309. [Google Scholar] [CrossRef]
- Stepanenko, I.N.; Novak, M.S.; Mühlgassner, G.; Roller, A.; Hejl, M.; Arion, V.B.; Jakupec, M.A.; Keppler, B.K. Organometallic 3-(1H-Benzimidazol-2-yl)-1H-pyazolo[3,4-b]pyridines as Potential Anticancer Agents. Inorg. Chem. 2011, 50, 11715–11728. [Google Scholar] [CrossRef]
- Ye, Q.; Cao, J.; Zhou, X.; Lv, D.; He, Q.; Yang, B.; Hu, Y. Synthesis and evaluation of novel 7-azaindazolyl-indolyl-maleimide derivatives as antitumor agents and protein kinase C inhibitors. Bioorg. Med. Chem. 2009, 17, 4763–4772. [Google Scholar] [CrossRef]
- Huang, P.-H.; Wen, Y.-S.; Shen, J.-Y. 3-Iodo-1H-pyazolo[3,4-b]pyridine. Acta Crystallogr. Sect. E 2014, 70, 650. [Google Scholar] [CrossRef] [Green Version]
- Nagender, P.; Malla Reddy, G.; Naresh Kumar, R.; Poornachandra, Y.; Ganesh Kumar, C.; Narsaiah, B. Synthesis, cytotoxicity, antimicrobial and anti-biofilm activities of novel pyazolo[3,4-b]pyridine and pyrimidine functionalized 1,2,3-triazole derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 2905–2908. [Google Scholar] [CrossRef] [PubMed]
- Nagender, P.; Naresh Kumar, R.; Malla Reddy, G.; Krishna Swaroop, D.; Poornachandra, Y.; Ganesh Kumar, C.; Narsaiah, B. Synthesis of novel hydrazone and azole functionalized pyazolo[3,4-b]pyridine derivatives as promising anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 4427–4432. [Google Scholar] [CrossRef] [PubMed]







| Class-I |  |
| Class-II |  |
| Class-III |  |
| Class-IV |  |
| S. No. | Compound | Physico-Chemical Properties | In vitro studies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone of Inhibition | MIC and MBC | ||||||||||||
| MW | iLogP | HA | HD | RB | TPSA | S. aureus | K. pneumoniae | S. aureus | K. pneumoniae | ||||
| MIC | MBC | MIC | MBC | ||||||||||
| 1. | 14 | 304.35 | 2.87 | 4 | 0 | 3 | 61.42 | NI | 11 ± 0.15 | 0.25 | 0.5 | 0.25 | 0.5 |
| 2. | 15 | 338.79 | 3.08 | 4 | 0 | 3 | 61.42 | 11 ± 0.12 | 13 ± 0.28 | 0.25 | 0.5 | 0.25 | 0.5 |
| 3. | 16 | 450.66 | 3.04 | 4 | 0 | 3 | 61.42 | 15 ± 0.59 | 12 ± 0.22 | 0.25 | 0.5 | 0.25 | 0.5 |
| 4. | 17 | 308.31 | 2.7 | 5 | 0 | 3 | 61.42 | NI | 11 ± 0.19 | 0.25 | 0.5 | 0.25 | 0.5 |
| 5. | 18 | 324.77 | 2.95 | 4 | 0 | 3 | 61.42 | NI | 11 ± 0.09 | 0.5 | 1.0 | 0.5 | 1.0 |
| 6. | 19 | 417.69 | 3.67 | 4 | 0 | 3 | 61.42 | 11 ± 0.17 | 13 ± 0.41 | 0.25 | 0.5 | 0.25 | 0.5 |
| 7. | 20 | 387.21 | 3.18 | 5 | 0 | 3 | 61.42 | NI | 13 ± 0.39 | 0.25 | 0.5 | 0.25 | 0.5 |
| 8. | 21 | 403.66 | 3.34 | 4 | 0 | 3 | 61.42 | 12 ± 0.19 | NI | 0.5 | 1.0 | 0.25 | 0.5 |
| 9. | 22 | 403.66 | 3.3 | 4 | 0 | 3 | 61.42 | 11 ± 0.09 | NI | 0.5 | 1.0 | 0.5 | 1.0 |
| 10. | 23 | 403.66 | 3.3 | 4 | 0 | 3 | 61.42 | 11 ± 0.16 | 10 ± 0.12 | 0.25 | 0.5 | 0.25 | 0.5 |
| 11. | 24 | 416.22 | 2.87 | 4 | 0 | 3 | 61.42 | 15 ± 0.82 | 14 ± 0.75 | 0.25 | 0.5 | 0.25 | 0.5 |
| 12. | 25 | 420.18 | 3.02 | 5 | 0 | 3 | 61.42 | 12 ± 0.18 | 15 ± 0.65 | 0.25 | 0.5 | 0.25 | 0.5 |
| 13. | 26 | 562.53 | 3.26 | 4 | 0 | 3 | 61.42 | 13 ± 0.27 | 14 ± 0.45 | 0.25 | 0.5 | 0.25 | 0.5 |
| 14. | 27 | 436.64 | 3.06 | 4 | 0 | 3 | 61.42 | 18 ± 0.95 | 16 ± 0.82 | 0.25 | 0.5 | 0.25 | 0.5 |
| 15. | 28 | 574.42 | 4.63 | 8 | 0 | 8 | 118.43 | 10 ± 0.15 | 11 ± 0.15 | 0.5 | 1.0 | 0.25 | 0.5 |
| 16. | 29 | 513.46 | 4.08 | 10 | 0 | 8 | 118.43 | 12 ± 0.21 | 14 ± 0.28 | 0.25 | 0.5 | 0.25 | 0.5 |
| 17. | 30 | 798.16 | 4.71 | 8 | 0 | 8 | 118.43 | 10 ± 0.13 | NI | 0.25 | 0.5 | 0.25 | 0.5 |
| 18. | 31 | 546.37 | 4.51 | 8 | 0 | 8 | 118.43 | 12 ± 0.19 | NI | 0.25 | 0.5 | 0.25 | 0.5 |
| 19. | Ciprofloxacin | 32 ± 0.40 | 31 ± 0.20 | 0.062 | 0.125 | 0.031 | 0.062 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandaru, N.R.; Makam, P.; Akshinthala, P.; Katari, N.K.; Banoth, V.; Kolli, B.; Gundla, R. Molecular Hybrids of Pyazolo[3,4-b]pyridine and Triazole: Design, Synthesis and In Vitro Antibacterial Studies. Molecules 2022, 27, 7647. https://doi.org/10.3390/molecules27217647
Bandaru NR, Makam P, Akshinthala P, Katari NK, Banoth V, Kolli B, Gundla R. Molecular Hybrids of Pyazolo[3,4-b]pyridine and Triazole: Design, Synthesis and In Vitro Antibacterial Studies. Molecules. 2022; 27(21):7647. https://doi.org/10.3390/molecules27217647
Chicago/Turabian StyleBandaru, Narasimha Rao, Parameshwar Makam, Parameswari Akshinthala, Naresh Kumar Katari, Venkanna Banoth, Balakrishna Kolli, and Rambabu Gundla. 2022. "Molecular Hybrids of Pyazolo[3,4-b]pyridine and Triazole: Design, Synthesis and In Vitro Antibacterial Studies" Molecules 27, no. 21: 7647. https://doi.org/10.3390/molecules27217647