Unusual Formation of 1,2,4-Oxadiazine Core in Reaction of Amidoximes with Maleic or Fumaric Esters
Abstract
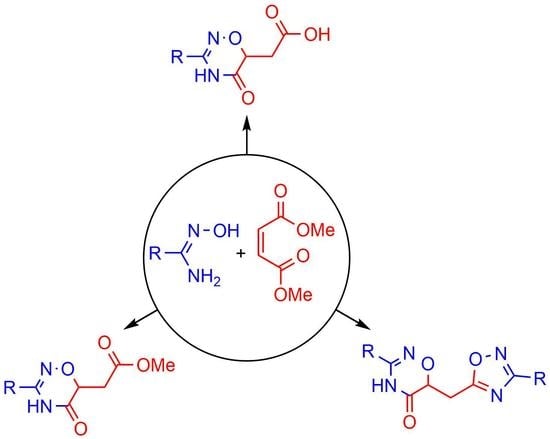
:1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. General
3.2. Oxadiazinones Preparation and Characterization
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Baykov, S.; Semenov, A.; Tarasenko, M.; Boyarskiy, V.P. Application of amidoximes for the heterocycles synthesis. Tetrahedron Lett. 2020, 61, 152403. [Google Scholar] [CrossRef]
- Mehmood, H.; Iqbal, M.A.; Lu, L.; Hua, R. Base-Promoted Annulation of Amidoximes with Alkynes: Simple Access to 2,4-Disubstituted Imidazoles. Molecules 2020, 25, 3621. [Google Scholar] [CrossRef] [PubMed]
- Pivneva, E.E.; Galenko, A.V.; Dar’In, D.V.; Lobanov, P.S. Rearrangement of the adducts of α-(aminocarbonyl)-acetamidoximes with acylacetylenes, leading to 2-aminopyrrole derivatives. Chem. Heterocycl. Compd. 2012, 48, 875–880. [Google Scholar] [CrossRef]
- Shabalin, D.A.; Dunsford, J.J.; Ngwerume, S.; Saunders, A.R.; Gill, D.M.; Camp, J.E. Synthesis of 2,4-Disubstituted Imidazoles via Nucleophilic Catalysis. Synlett 2020, 31, 797–800. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Bokach, N.A.; Demakova, M.Y.; Kukushkin, V.Y. Metal-Involving Synthesis and Reactions of Oximes. Chem. Rev. 2017, 117, 13039–13122. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Bokach, N.A.; Kukushkin, V.Y. Coordination chemistry and metal-involving reactions of amidoximes: Relevance to the chemistry of oximes and oxime ligands. Coord. Chem. Rev. 2016, 313, 62–93. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xu, H.; Xu, Y.; Ding, T.; Zhang, W.; Ren, Y.; Chang, H. Base-mediated one-pot synthesis of 1,2,4-oxadiazoles from nitriles, aldehydes and hydroxylamine hydrochloride without addition of extra oxidant. Org. Biomol. Chem. 2016, 14, 9814–9822. [Google Scholar] [CrossRef]
- Shetnev, A.A.; Pankratieva, V.E.; Kunichkina, A.S.; Vlasov, A.S.; Proskurina, I.K.; Kotov, A.; Korsakov, M.K. Synthesis of 3,5-Disubstituted 1,2,4-Oxadiazoles from Amidoximes and Aldehydes in the Superbasic System NaOH/DMSO. Russ. J. Org. Chem. 2020, 56, 1181–1186. [Google Scholar] [CrossRef]
- Teslenko, F.E.; Churakov, A.I.; Larin, A.A.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Route to 1,2,4- and 1,2,5-oxadiazole ring assemblies via a one-pot condensation/oxidation protocol. Tetrahedron Lett. 2020, 61, 151678. [Google Scholar] [CrossRef]
- Sharonova, T.; Pankrat’Eva, V.; Savko, P.; Baykov, S.; Shetnev, A. Facile room-temperature assembly of the 1,2,4-oxadiazole core from readily available amidoximes and carboxylic acids. Tetrahedron Lett. 2018, 59, 2824–2827. [Google Scholar] [CrossRef]
- Thacker, P.S.; Angeli, A.; Argulwar, O.S.; Tiwari, P.L.; Arifuddin, M.; Supuran, C.T. Design, synthesis and biological evaluation of coumarin linked 1,2,4-oxadiazoles as selective carbonic anhydrase IX and XII inhibitors. Bioorg. Chem. 2020, 98, 103739. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, A.; Bogolubsky, A.V.; Pipko, S.E.; Grishchenko, A.V.; Ushakov, D.V.; Zhemera, A.V.; Viniychuk, O.O.; Konovets, A.I.; Zaporozhets, O.A.; Mykhailiuk, P.K.; et al. Expanding Synthesizable Space of Disubstituted 1,2,4-Oxadiazoles. ACS Comb. Sci. 2016, 18, 616–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarasinghe, K.K.; Maier, M.B.; Srivastava, A.; Gray, J.L. One-pot synthesis of 1,2,4-oxadiazoles from carboxylic acid esters and amidoximes using potassium carbonate. Tetrahedron Lett. 2006, 47, 3629–3631. [Google Scholar] [CrossRef]
- Strelnikova, J.; Rostovskii, N.V.; Starova, G.L.; Khlebnikov, A.F.; Novikov, M.S. Rh(II)-Catalyzed Transannulation of 1,2,4-Oxadiazole Derivatives with 1-Sulfonyl-1,2,3-triazoles: Regioselective Synthesis of 5-Sulfonamidoimidazoles. J. Org. Chem. 2018, 83, 11232–11244. [Google Scholar] [CrossRef] [PubMed]
- Shetnev, A.; Baykov, S.; Kalinin, S.; Belova, A.; Sharoyko, V.; Rozhkov, A.; Zelenkov, L.; Tarasenko, M.; Sadykov, E.; Korsakov, M.; et al. 1,2,4-Oxadiazole/2-Imidazoline Hybrids: Multi-target-directed Compounds for the Treatment of Infectious Diseases and Cancer. Int. J. Mol. Sci. 2019, 20, 1699. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.; Na, H.G.; Oh, E.H.; Jung, E.; Lee, Y.H.; Jeong, E.J.; Ou, Y.-D.; Zhou, B.; Ahn, S.; Shin, J.S.; et al. Discovery and synthesis of 1,2,4-oxadiazole derivatives as novel inhibitors of Zika, dengue, Japanese encephalitis, and classical swine fever virus infections. Arch. Pharmacal Res. 2022, 45, 280–293. [Google Scholar] [CrossRef]
- Tarasenko, M.; Duderin, N.; Sharonova, T.; Baykov, S.; Shetnev, A.; Smirnov, A.V. Room-temperature synthesis of pharmaceutically important carboxylic acids bearing the 1,2,4-oxadiazole moiety. Tetrahedron Lett. 2017, 58, 3672–3677. [Google Scholar] [CrossRef]
- Baykov, S.; Tarasenko, M.; Zelenkov, L.E.; Kasatkina, S.; Savko, P.; Shetnev, A. Diastereoselective Opening of Bridged Anhydrides by Amidoximes Providing Access to 1,2,4-Oxadiazole/Norborna(e)ne Hybrids. Eur. J. Org. Chem. 2019, 2019, 5685–5693. [Google Scholar] [CrossRef]
- Presnukhina, S.; Tarasenko, M.; Baykov, S.; Smirnov, S.N.; Boyarskiy, V.; Shetnev, A.; Korsakov, M.K. Entry into (E)-3-(1,2,4-oxadiazol-5-yl)acrylic acids via a one-pot ring-opening/ring-closing/retro-Diels-Alder reaction sequence. Tetrahedron Lett. 2020, 61, 151543. [Google Scholar] [CrossRef]
- Tarasenko, M.V.; Kotlyarova, V.D.; Baykov, S.V.; Shetnev, A.A. 2-(1,2,4-Oxadiazol-5-yl)anilines Based on Amidoximes and Isatoic Anhydrides: Synthesis and Structure Features. Russ. J. Gen. Chem. 2021, 91, 768–778. [Google Scholar] [CrossRef]
- Tsiulin, P.A.; Sosnina, V.V.; Krasovskaya, G.G.; Danilova, A.S.; Baikov, S.V.; Kofanov, E. Formation and cyclization of N′-(benzoyloxy)benzenecarboximidamides. Russ. J. Org. Chem. 2011, 47, 1874–1877. [Google Scholar] [CrossRef]
- Novikov, M.S.; Strelnikova, J.O.; Rostovskii, N.V.; Khoroshilova, O.V.; Khlebnikov, A.F. An Efficient Synthesis of Functionalized 2H-1,3,5-Oxadiazines via Metal-Carbenoid-Induced 1,2,4-Oxadiazole Ring Cleavage. Synthesis 2021, 53, 348–358. [Google Scholar] [CrossRef]
- Agat’Ev, P.A.; Shlenev, R.M.; Tarasov, A.V.; Danilova, A.S. New synthesis of 3,5-diaryl-1,2,4-oxadiazoles containing a sulfonyl chloride or sulfonamide group. Russ. J. Org. Chem. 2015, 51, 988–991. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Ananyev, I.V.; Makhova, N.N. Efficient assembly of mono- and bis(1,2,4-oxadiazol-3-yl)furoxan scaffolds via tandem reactions of furoxanylamidoximes. RSC Adv. 2015, 5, 47248–47260. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, X.; Chen, B. Direct synthesis of 2,4,5-trisubstituted imidazoles and di/tri-substituted pyrimidines via cycloadditions of α,β-unsaturated ketones/aldehydes and N′-hydroxyl imidamides. Tetrahedron Lett. 2019, 60, 1103–1107. [Google Scholar] [CrossRef]
- Dénes, L.; Jednákovits, A.; Hargitai, J.; Pénzes, Z.; Balla, A.; Tálosi, L.; Krajcsi, P.; Csermely, P. Pharmacologically activated migration of aortic endothelial cells is mediated through p38 SAPK. J. Cereb. Blood Flow Metab. 2002, 136, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Weller, H.N.; Miller, A.V.; Dickinson, K.E.J.; Hedberg, S.A.; Delaney, C.L.; Serafino, R.P.; Moreland, S. Synthesis of N-Alkyl-1,2,4-oxadiazinones as Angiotensin-II (AT1) Receptor Antagonists. Heterocycles 1993, 36, 1027–1038. [Google Scholar] [CrossRef]
- Bursavich, M.G.; Harrison, B.A.; Acharya, R.; Costa, D.E.; Freeman, E.A.; Hrdlicka, L.A.; Jin, H.; Kapadnis, S.; Moffit, J.S.; Murphy, D.; et al. Discovery of the Oxadiazine FRM-024: A Potent CNS-Penetrant Gamma Secretase Modulator. J. Med. Chem. 2021, 64, 14426–14447. [Google Scholar] [CrossRef]
- Bursavich, M.G.; Harrison, B.A.; Acharya, R.; Costa, D.E.; Freeman, E.A.; Hodgdon, H.E.; Hrdlicka, L.A.; Jin, H.; Kapadnis, S.; Moffit, J.S.; et al. Design, Synthesis, and Evaluation of a Novel Series of Oxadiazine Gamma Secretase Modulators for Familial Alzheimer’s Disease. J. Med. Chem. 2017, 60, 2383–2400. [Google Scholar] [CrossRef]
- Hakimelahi, G.H.; Li, P.-C.; Moosavi-Movahedi, A.A.; Chamani, J.; Khodarahmi, G.A.; Ly, T.W.; Valiyev, F.; Leong, M.K.; Hakimelahi, S.; Shia, K.-S.; et al. Application of the Barton photochemical reaction in the synthesis of 1-dethia-3-aza-1-carba-2-oxacephem: A novel agent against resistant pathogenic microorganisms. Org. Biomol. Chem. 2003, 1, 2461–2467. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, W.; Liu, X.; Li, H.; Sun, G.; Mandal, M.; Vicarel, M.; Zhu, X.; Bennett, C.; McCraken, T.; et al. Synthesis and SAR Studies of Fused Oxadiazines as γ-Secretase Modulators for Treatment of Alzheimer’s Disease. ACS Med. Chem. Lett. 2012, 3, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qin, J.; Dhondi, P.; Zhou, W.; Vicarel, M.; Bara, T.; Cole, D.; Josien, H.; Pissarnitski, D.; Zhu, Z.; et al. The discovery of fused oxadiazepines as gamma secretase modulators for treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2013, 23, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-Y.; Asberom, T.; Bara, T.; Bennett, C.; Burnett, D.; Chu, I.; Clader, J.; Cohen-Williams, M.; Cole, D.; Czarniecki, M.; et al. Cyclic Hydroxyamidines as Amide Isosteres: Discovery of Oxadiazolines and Oxadiazines as Potent and Highly Efficacious γ-Secretase Modulators in Vivo. J. Med. Chem. 2012, 55, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Shetnev, A.A.; Zubkov, F.I. The latest advances in chemistry of 1,2,4-oxadiazines (microreview). Chem. Heterocycl. Compd. 2017, 53, 495–497. [Google Scholar] [CrossRef]
- Kara, Y.S.; Yıldız, B. Synthesis and substituent effect study on 13C NMR chemical shifts of 4-(substitue-phenyl)-6-methyl-3-phenyl-4H-1,2,4-oxadiazin-5(6H)-one. J. Mol. Struct. 2022, 1250, 131787. [Google Scholar] [CrossRef]
- Dürüst, Y.; Altuğ, C.; Kiliç, F. Thiophene-Substituted 1,2,4-Oxadiazoles and Oxadiazines. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 299–313. [Google Scholar] [CrossRef]
- Veerman, J.J.N.; Bursavich, M.G.; Bruseker, Y.B.; van Esseveldt, B.C.J.; Glen, R.; Harrison, B.A.; Heijne, E.H.; McRiner, A.J.; Meulemans, T.M.; van Rijnsbergen, P.; et al. Strategic and Tactical Approaches to the Synthesis of 5,6-Dihydro-[1,2,4]oxadiazines. Heterocycles 2016, 92, 2166–2200. [Google Scholar] [CrossRef]
- Ranjbar-Karimi, R.; Karbakhsh-Ravari, A.; Poorfreidoni, A. Reactions of pentafluoropyridine with amidoximes. J. Iran. Chem. Soc. 2017, 14, 2397–2405. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Lin, C.-D.; Wang, J.-H.; Wen, L.-R. One Base for Two Shots: Metal-Free Substituent-Controlled Synthesis of Two Kinds of Oxadiazine Derivatives from Alkynylbenziodoxolones and Amidoximes. J. Org. Chem. 2019, 84, 6904–6915. [Google Scholar] [CrossRef]
- Guzmán, A.; Romero, S.; Urquiza, E.M.G.; Muchowski, J.M. Reaction of arylamidoximes with dimethyl acetylenedicarboxylate and diethyl chlorofumarate. Stereochemistry of the adducts and the derived 1,2,4-oxadiazines. J. Heterocycl. Chem. 1980, 17, 1101–1105. [Google Scholar] [CrossRef]
- Baykov, S.; Sharonova, T.; Osipyan, A.; Rozhkov, S.; Shetnev, A.; Smirnov, A. A convenient and mild method for 1,2,4-oxadiazole preparation: Cyclodehydration of O-acylamidoximes in the superbase system MOH/DMSO. Tetrahedron Lett. 2016, 57, 2898–2900. [Google Scholar] [CrossRef]
- Tarasenko, M.; Sidneva, V.; Belova, A.; Romanycheva, A.; Sharonova, T.; Baykov, S.; Shetnev, A.; Kofanov, E.; Kuznetsov, M. An efficient synthesis and antimicrobial evaluation of 5-alkenyl- and 5-styryl-1,2,4-oxadiazoles. Arkivoc 2018, 2018, 458–470. [Google Scholar] [CrossRef]
- Baykov, S.; Sharonova, T.; Shetnev, A.; Rozhkov, S.; Kalinin, S.; Smirnov, A.V. The first one-pot ambient-temperature synthesis of 1,2,4-oxadiazoles from amidoximes and carboxylic acid esters. Tetrahedron 2017, 73, 945–951. [Google Scholar] [CrossRef]
- Zora, M.; Kivrak, A.; Kelgokmen, Y. A novel one-pot synthesis of ferrocenyl-substituted 1,2,4-oxadiazoles. J. Organomet. Chem. 2014, 759, 67–73. [Google Scholar] [CrossRef]
- Gangloff, A.R.; Litvak, J.; Shelton, E.J.; Sperandio, D.; Wang, V.R.; Rice, K.D. Synthesis of 3,5-disubstituted-1,2,4-oxadiazoles using tetrabutylammonium fluoride as a mild and efficient catalyst. Tetrahedron Lett. 2001, 42, 1441–1443. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Pereira, M.C.; Faustino, W.W.M.; Coutinho, K.; dos Anjos, J.V.; De Melo, S.J. Synthesis, mechanism of formation, and molecular orbital calculations of arylamidoximes. Mon. Chem.—Chem. Mon. 2009, 140, 1319. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CrysAlis Pro. Data Collection and Processing Software for Agilent X-ray Diffractometers; Aglient Technologies: Yarnton, UK, 2013. [Google Scholar]





 | ||||||
|---|---|---|---|---|---|---|
| Entry | 2a (Equiv) | Base (Equiv) | Time, h | Isolated Yield, % | ||
| 3a | 4a | 5a | ||||
| 1 | 1.5 | NaOH (1.5) | 4 | 37 | 26 | – |
| 2 | 2.0 | NaOH (2.0) | 4 | 47 | 17 | – |
| 3 | 2.5 | NaOH (2.5) | 4 | 35 | 14 | – |
| 4 | 2.0 | NaOH (2.0) | 18 | 65 | trace | – |
| 5 | 2.0 | NaOH (2.0) | 24 | 61 | trace | – |
| 6 | 2.0 | LiOH (2.0) | 18 | 60 | trace | – |
| 7 | 2.0 | KOH (2.0) | 18 | 55 | trace | – |
| 8 | 2.0 | t-BuONa (1.0) | 4 | – | 78 | trace |
| 9 | 2.0 | t-BuONa (2.0) | 4 | – | 58 | 18 |
| 10 | 0.4 | t-BuONa (2.0) | 4 | – | 29 | 54 |
| 11 | 0.4 | t-BuONa (2.0) | 18 | – | trace | 82 |
| 12 a | 2.0 | NaOH (2.0) | 18 | 63 | trace | – |
| 13 a | 2.0 | t-BuONa (1.0) | 4 | – | 76 | trace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presnukhina, S.I.; Tarasenko, M.V.; Geyl, K.K.; Baykova, S.O.; Baykov, S.V.; Shetnev, A.A.; Boyarskiy, V.P. Unusual Formation of 1,2,4-Oxadiazine Core in Reaction of Amidoximes with Maleic or Fumaric Esters. Molecules 2022, 27, 7508. https://doi.org/10.3390/molecules27217508
Presnukhina SI, Tarasenko MV, Geyl KK, Baykova SO, Baykov SV, Shetnev AA, Boyarskiy VP. Unusual Formation of 1,2,4-Oxadiazine Core in Reaction of Amidoximes with Maleic or Fumaric Esters. Molecules. 2022; 27(21):7508. https://doi.org/10.3390/molecules27217508
Chicago/Turabian StylePresnukhina, Sofia I., Marina V. Tarasenko, Kirill K. Geyl, Svetlana O. Baykova, Sergey V. Baykov, Anton A. Shetnev, and Vadim P. Boyarskiy. 2022. "Unusual Formation of 1,2,4-Oxadiazine Core in Reaction of Amidoximes with Maleic or Fumaric Esters" Molecules 27, no. 21: 7508. https://doi.org/10.3390/molecules27217508